Association between levothyroxine supplementation for hypothyroidism in late pregnancy and risk of prematurity: a population-based cohort study
- PMID: 39985026
- PMCID: PMC11846315
- DOI: 10.1186/s12916-025-03934-1
Association between levothyroxine supplementation for hypothyroidism in late pregnancy and risk of prematurity: a population-based cohort study
Abstract
Background: Hypothyroidism in pregnancy is associated with obstetrical and fetal complications, such as prematurity. However, whether its management by levothyroxine affects the risk of prematurity is not yet clear.
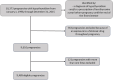
Methods: We conducted a cohort study within the Quebec Pregnancy Cohort including pregnancies with hypothyroidism from January 1, 1998, through December 31, 2015. In primary analyses, we considered levothyroxine exposure (yes/no), total duration, mean daily dose, and cumulative dose in the 2-months period before delivery (for preterm deliveries) or before 37th weeks' gestation (for term deliveries). Secondly, levothyroxine dosage before and after the beginning of the second trimester were compared, and pregnancies were categorized in increased or constant dosage groups. Lastly, levothyroxine was also defined as a time-varying daily exposure from the 14th weeks' gestation until delivery or 37th weeks' gestation, whichever came first. Prematurity was defined as giving birth before the 37th weeks' gestation. Term pregnancies were censored at 37th weeks' gestation because they were no longer at risk of prematurity afterwards. Generalized estimating equations and Cox-proportional hazard models, adjusted for potential confounders, were used to calculate adjusted relative risks (aRRs) and hazard ratios (aHRs), respectively.
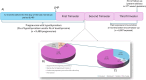
Results: A total of 9489 pregnant individuals with hypothyroidism were included. Among them, 6667 (70.3%) were exposed to levothyroxine in the 2-months time-window. Adjusting for potential confounders, no association was observed between levothyroxine exposure (aRR, 0.98; 95% CI, 0.81-1.20) and the risk of prematurity compared to non-exposed. Also, no association between levothyroxine duration (> 30 days: aRR, 0.99; 95% CI, 0.81-1.21), cumulative dose (> 7125 mcg: aRR, 0.97; 95% CI, 0.73-1.27) or mean daily dose (> 125 mcg/day: aRR, 0.95; 95% CI, 0.72-1.26) and the risk of prematurity was observed, compared to non-exposure. Finally, the risk of prematurity did not vary between increased or constant dosage groups (aRR, 0.84; 95% CI, 0.67-1.05). Similarly, time-varying exposure analysis did not show any association between levothyroxine exposure and prematurity risk (aHR, 0.95; 95% CI, 0.81-1.11).
Conclusions: Levothyroxine supplementation in late pregnancy among individuals with hypothyroidism was not associated with prematurity risk. Our findings support the safe use of levothyroxine during gestation and might be useful for the current guidelines.
Keywords: Hypothyroidism; Levothyroxine; Pregnancy; Prematurity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate/publication: The acquisition of the data for the creation of the Quebec Pregnancy Cohort was approved by the Ethics Review Board of Centre Hospitalier Universaire Sainte-Justine, Montreal (reference numbers: 1740 and 2976). Additionally, the Commission d’accès à l’information authorized the linkage of databases (reference number: 1005446-S). The study was conducted in compliance with Canadian research regulations. Administrative data from the provincial health systems of Quebec were used for the study. All data were anonymized to ensure the confidentiality of individuals, and no personally identifiable information is accessible or shareable. Only aggregate data resulting from analyses will be published. Consequently, informed consent from individual participants was not required for this study. Competing interests: The authors declare no competing interests.
Figures
References
-
- Tsakiridis I, Giouleka S, Kourtis A, Mamopoulos A, Athanasiadis A, Dagklis T. Thyroid disease in pregnancy: a descriptive review of guidelines. Obstet Gynecol Surv. 2022;77(1):45–62. - PubMed
-
- Thyroid Disease in Pregnancy: ACOG Practice Bulletin, Number 223. Obstet Gynecol. 2020;135(6):e261–e274. 10.1097/AOG.0000000000003893. - PubMed
-
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–89. - PubMed
-
- Stagnaro-Green A, Pearce E. Thyroid disorders in pregnancy. Nat Rev Endocrinol. 2012;8(11):650–8. - PubMed
-
- Yazbeck CF, Sullivan SD. Thyroid disorders during pregnancy. Med Clin North Am. 2012;96(2):235–56. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

