The extracellular matrix protein type I collagen and fibronectin are regulated by β-arrestin-1/endothelin axis in human ovarian fibroblasts
- PMID: 39985042
- PMCID: PMC11844176
- DOI: 10.1186/s13046-025-03327-5
The extracellular matrix protein type I collagen and fibronectin are regulated by β-arrestin-1/endothelin axis in human ovarian fibroblasts
Abstract
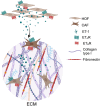
Background: The invasive and metastatic spread of serous ovarian cancer (SOC) results from the cooperative interactions between cancer and stroma, which include extracellular matrix (ECM) and cellular components, including cancer-associated fibroblasts (CAFs). Soluble factors secreted by cancer and stromal cells contribute to stroma remodeling through the secretion of ECM proteins, providing a favorable environment for cancer cell dissemination. The peptide endothelin-1 (ET-1), through two G protein-coupled receptors (GPCR), endothelin receptor type A (ETAR) and B (ETBR), acts on both cancer and stromal cells, engaging the protein β-arrestin1 (β-arr1), to bolster SOC progression. However, its role in the regulation of the ECM proteins by ovarian fibroblasts is not understood. This study delves into the role of ET-1 as a regulator of type I collagen (Col1) and fibronectin (FN).
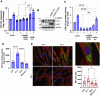
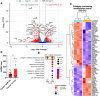
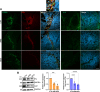
Methods: We used human primary ovarian fibroblasts (HOFs) and CAFs. The expression of Col1 (COL1A1) and FN (FN1) were detected by western blotting (WB), quantitative real time-polymerase chain reaction (qRT-PCR), immunofluorescence (IF), and confocal laser scanning microscopy (CLSM) in cells and tumor tissue sections from mice xenografts, while the transcription of COL1A1 was detected by luciferase reporter gene assay. The nuclear function of β-arr1 was evaluated by silencing and rescue expression with wild-type (WT) and nuclear mutant plasmid constructs, RNA seq and differential gene expression and gene sets enrichment analyses. The prognostic role of COL1A1, FN1, EDN1 (ET-1) and ARRB1 (β-arr1) gene expression was evaluated using the Kaplan-Meier plotter database and clinical ovarian cancer tissue samples.
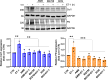
Results: We demonstrated that ET-1 boosts Col1 and FN expression in HOFs, akin to ovarian CAF levels. Both receptors are implicated, evident from inhibitory effects after ETAR or ETBR antagonist treatments and notably with bosentan, a dual antagonist, in vitro and in vivo. At the molecular level, ET-1 triggers the activation of COL1A1 promoter activity and its enhanced expression via β-arr1 nuclear function. Transcriptome analysis of β-arr1-silenced HOFs confirms the nuclear role of β-arr1 in collagen and ECM remodeling-related protein transcriptional regulation. Accordingly, a high level of EDN1/ARRB1 expression in combination with either COL1A1 or FN1 is associated with the poor prognosis of SOC patients.
Conclusions: These findings hint at ET-1 involvement in ECM remodeling and early SOC stages by modulating the expression of Col1 and FN. Targeting ET-1 signaling with ETAR/ETBR antagonists might interfere with the ability of CAFs to produce key ECM proteins in this tumor.
Keywords: Endothelin receptors; Endothelin-1; Fibroblasts; Fibronectin; Ovarian cancer; Type I collagen; β-arrestin 1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: For in vivo animal studies, the experimental protocols complied with the principles of ARRIVE ( https://arriveguidelines.org ) according to institutional guidelines and the Italian Law (D-lgs 26/2014) and were approved by the National Ethics Committee for Animal Experimentation of the Italian Ministry of Health (authorization N1/2020- PR #365869604 Responsible Researcher Dr Laura Rosanò). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Rosanò L, Spinella F, Bagnato A. Endothelin 1 in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2013;13:637–51. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

