Discarded intravenous medication in the ICU: the GAME-OVER multicenter prospective observational study
- PMID: 39985053
- PMCID: PMC11846455
- DOI: 10.1186/s13054-025-05299-6
Discarded intravenous medication in the ICU: the GAME-OVER multicenter prospective observational study
Abstract
Background: Medication waste is a contributor to the healthcare environmental footprint and impacts ecosystems. Data on medication waste in the intensive care unit (ICU) are scarce, and therefore are essential to develop new sustainable strategies.
Methods: The GAME-OVER French multicenter prospective observational study was conducted from November 2022 to March 2023, over a 24-h period of choice, at the discretion of each participating center. Adult ICUs were enrolled in the study on a voluntary basis and hospitalized patients who did not express opposition were included in the analysis. The primary endpoint was the percentage of discarded intravenous (IV) medication in the ICU, defined as the ratio of the discarded volume to the total volume of IV medication prepared. Secondary endpoints included identifying risk factors and main reasons for medication waste and estimating its related healthcare cost.
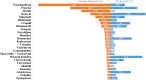
Results: Among the 81 ICUs and the 1076 enrolled patients, 408.9 L of 130 IV medications were prepared. The discarded volume was 43.8 L, resulting in a 10.7% discarded IV medication (95% Confidence Interval (CI), 9.9-11.5). Number of daily admissions/discharges in the ICU, as admission for elective surgery, Sequential Organ Failure Assessment score ≥ 7, endotracheal intubation, renal replacement therapy and body mass index were independently associated with increased discarded IV medication. Ninety percent of pharmaceutical waste was attributed to 25 key drugs, with an estimated national annual cost of 2,737,163€.
Conclusions: Discarded intravenous medication in the ICU is considerable and results in significant costs for the health care system, without obvious patient-centered value. Risk factors associated with medication waste were largely nonmodifiable, emphasizing the need for sustainable practices in patient care and resource management.
Trial registration: ClinicalTrials.gov: NCT05553054 . September 23, 2022.
Keywords: Drug waste; Ecodesign of healthcare; Environment; Healthcare cost; Pharmaceutical waste; Sustainable healthcare.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The protocol was approved by an IRB on September 15, 2022 (Centre Hospitalier Intercommunal de Toulon – La Seyne-sur-Mer, France, IRB 12962). The study did not involve clinical follow-up. The database was approved by the sponsor’s Data Protection Officer, in accordance with the European Union general data protection regulation. In accordance with French law, all the adult patients hospitalized in the participating ICU during the day of inclusion, or their next of kin if not able to consent, were informed of the study protocol and included if they did not express opposition to data collection. Consent for publication: Not applicable. Competing interest: SP received a mobility research grant from L’Institut Servier, outside of the submitted work.
Figures

References
-
- Howard C, MacNeill AJ, Hughes F, Alqodmani L, Charlesworth K, de Almeida R, et al. Learning to treat the climate emergency together: social tipping interventions by the health community. Lancet Planet Health. 2023;7:e251–64. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

