Neuronal TDP-43 aggregation drives changes in microglial morphology prior to immunophenotype in amyotrophic lateral sclerosis
- PMID: 39985110
- PMCID: PMC11844090
- DOI: 10.1186/s40478-025-01941-0
Neuronal TDP-43 aggregation drives changes in microglial morphology prior to immunophenotype in amyotrophic lateral sclerosis
Abstract
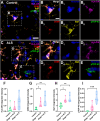
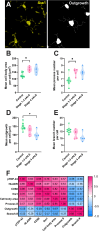
Microglia are the innate immune cells of the brain with the capacity to react to damage or disease. Microglial reactions can be characterised in post-mortem tissues by assessing their pattern of protein expression, or immunophenotypes, and cell morphologies. We recently demonstrated that microglia have a phagocytic immunophenotype in early-stage ALS but transition to a dysfunctional immunophenotype by end stage, and that these states are driven by TAR DNA-binding protein 43 (TDP-43) aggregation in the human brain. However, it remains unclear how microglial morphologies are changed in ALS. Here we examine the relationship between microglial immunophenotypes and morphologies, and TDP-43 pathology in motor cortex tissue from people with ALS and from a TDP-43-driven ALS mouse model. Post-mortem human brain tissue from 10 control and 10 ALS cases was analysed alongside brain tissue from the bigenic NEFH-tTA/tetO-hTDP-43∆NLS (rNLS) mouse model of ALS at distinct disease stages. Sections were immunohistochemically labelled for microglial markers (HLA-DR, CD68, and Iba1) and phosphorylated TDP-43 (pTDP-43). Single-cell microglial HLA-DR, CD68, and Iba1 average intensities, and morphological features (cell body area, process number, total outgrowth, and branch number) were measured using custom image analysis pipelines. In human ALS motor cortex, we identified a significant change in microglial morphologies from ramified to hypertrophic, which was associated with increased Iba1 and CD68 levels. In the rNLS mouse motor cortex, the microglial morphologies changed from ramified to hypertrophic and increased Iba1 levels occurred in parallel with pTDP-43 aggregation, prior to increases in CD68 levels. Overall, the evidence presented in this study demonstrates that microglia change their morphologies prior to immunophenotype changes. These morphological changes may prime microglia near neurons with pTDP-43 aggregation for phagocytosis, in turn triggering immunophenotype changes; first, to a phagocytic state then to a dysfunctional one.
Keywords: Amyotrophic lateral sclerosis; CD68; Dystrophic; Hypertrophic; Iba1; Microglia; Ramified; TDP-43.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All human brain tissue was donated to the Neurological Foundation Human Brain Bank following donor and donor family consent, and its use in this study was approved by the University of Auckland Human Participants Ethics committee (protocol number 011654). Animal ethics approval was obtained from The University of Queensland (#QBI/131/18), and experiments were conducted in accordance with the Australian code of practice for the care and use of animals for scientific purposes. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Aderem A, Underhill DM (2002) Mechanisms of Phagocytosis in macrophages. Annu Rev Immunol 17:593–623 - PubMed
-
- Gibbons HM, Smith AM, Teoh HH, Bergin PM, Mee EW, Faull RLM et al (2011) Valproic acid induces microglial dysfunction, not apoptosis, in human glial cultures. Neurobiol Dis 41:96–103 - PubMed
-
- Rogers J, Strohmeyer R, Kovelowski CJ, Li R (2002) Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia 40:260–269 - PubMed
-
- Simard AR, Soulet D, Gowing G, Julien J-P, Rivest S (2006) Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49:489–502 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

