Spatiotemporal Transcriptomic Profiling Reveals the Dynamic Immunological Landscape of Alveolar Echinococcosis
- PMID: 39985260
- PMCID: PMC12079354
- DOI: 10.1002/advs.202405914
Spatiotemporal Transcriptomic Profiling Reveals the Dynamic Immunological Landscape of Alveolar Echinococcosis
Abstract
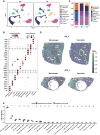
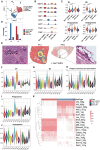
Alveolar echinococcosis (AE) is caused by the chronic infection of E. multilocularis, whose tumor-like growth can lead to high fatality if improperly treated. The early diagnosis of infection and the treatment of advanced AE remain challenging. Herein, bulk RNA-seq, scRNA-seq, and spatial transcriptomics technologies are integrated, to reveal the host immune response mechanism against E. multilocularis both spatially and chronologically, collecting mouse liver samples at multiple timepoints up to 15 months post infection. These results unveil an unprecedented high-resolution spatial atlas of the E. multilocularis infection foci and the functional roles of neutrophils, Spp1+ macrophages, and fibroblasts during disease progression. The heterogeneity of neutrophil and macrophage subpopulations are critical in both parasite-killing and the occurrence of immunosuppression during AE progression. These findings indicate the transition of parasite control strategy from "active killing" to "negative segregation" by the host, providing instructive insights into the treatment strategy for echinococcosis.
Keywords: Echinococcus multilocularis; macrophages; neutrophils; single‐cell RNA sequencing; spatial transcriptomics.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
A.C., S.L., and X.X. have patents associated with Stereo‐seq technology. The other authors declare no competing interests.
Figures





References
-
- Siegert S., Neumann S., J. Helminthol. 2022, 96, e45. - PubMed
-
- Deplazes P., Rinaldi L., Alvarez Rojas C. A., Torgerson P. R., Harandi M. F., Romig T., Antolova D., Schurer J. M., Lahmar S., Cringoli G., Magambo J., Thompson R. C., Jenkins E. J., Adv. Parasitol. 2017, 95, 315. - PubMed
-
- Moro P., Schantz P. M., Int. J. Infect. Dis. 2009, 13, 125. - PubMed
-
- Eckert J., Thompson R. C., Adv. Parasitol. 2017, 95, 1. - PubMed
MeSH terms
Supplementary concepts
Grants and funding
- 2022YFD1800200/National Key Research and Development Program
- 24JRRA807/Gansu Province Joint Research Fund
- 24ZD13NA008,23ZDNA007,22ZD6NA001/National Natural Science Foundation of China (32402915), The Major Science and Technology Project of Gansu Province
- CAAS-ASTIP-2021-LVRI,CAASTIP-2024-05/Innovation Program of Chinese Academy of Agricultural Sciences
- 2021-ZJ-933/Natural Science Foundation of Qinghai Province
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
