Fundamentals and Applications of Dual-Frequency Magnetic Particle Spectroscopy: Review for Biomedicine and Materials Characterization
- PMID: 39985275
- PMCID: PMC11967826
- DOI: 10.1002/advs.202416838
Fundamentals and Applications of Dual-Frequency Magnetic Particle Spectroscopy: Review for Biomedicine and Materials Characterization
Abstract
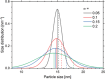
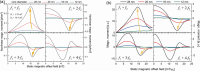
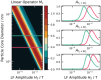
Superparamagnetic nanoparticles (MNP) offer exciting applications for engineering and biomedicine in imaging, diagnostics, and therapy upon magnetic excitation. Specifically, if excited at two distinct frequencies f1 and f2, MNP responds with magnetic intermodulation frequencies m·f1 ± n·f2 caused by their nonlinear magnetization. These mixing frequencies are highly specific for MNP properties, uniquely characterizing their presence. In this review, the fundamentals of frequency mixing magnetic detection (FMMD) as a special case of magnetic particle spectroscopy (MPS) are reviewed, elaborating its functional principle that enables a large dynamic range of detection of MNP. Mathematical descriptions derived from Langevin modeling and micromagnetic Monte-Carlo simulations show matching predictions. The latest applications of FMMD in nanomaterials characterization as well as diagnostic and therapeutic biomedicine are highlighted: analysis of the phase of the FMMD signal characterizes the magnetic relaxation of MNP, allowing to determine hydrodynamic size and binding state. Variation of excitation amplitudes or magnetic offset fields enables determining the size distribution of the particles' magnetic cores. This permits multiplex detection of polydisperse MNP in magnetic immunoassays, realized successfully for various biomolecular targets such as viruses, bacteria, proteins, and toxins. A portable magnetic reader enables portable immunodetection at point-of-care. Future applications toward theranostics are summarized and elaborated.
Keywords: magnetic biosensing; magnetic fluid hyperthermia (MFH); magnetic frequency mixing detection (FMMD); magnetic immunoassays; magnetic particle imaging (MPI); magnetic particle spectroscopy (MPS); micromagnetic simulation.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Key Contributors to Signal Generation in Frequency Mixing Magnetic Detection (FMMD): An In Silico Study.Sensors (Basel). 2024 Mar 18;24(6):1945. doi: 10.3390/s24061945. Sensors (Basel). 2024. PMID: 38544208 Free PMC article.
-
Comparative Modeling of Frequency Mixing Measurements of Magnetic Nanoparticles Using Micromagnetic Simulations and Langevin Theory.Nanomaterials (Basel). 2021 May 11;11(5):1257. doi: 10.3390/nano11051257. Nanomaterials (Basel). 2021. PMID: 34064640 Free PMC article.
-
Impact of Particle Size on the Nonlinear Magnetic Response of Iron Oxide Nanoparticles during Frequency Mixing Magnetic Detection.Sensors (Basel). 2024 Jun 29;24(13):4223. doi: 10.3390/s24134223. Sensors (Basel). 2024. PMID: 39001003 Free PMC article.
-
Combining magnetic particle imaging and magnetic fluid hyperthermia for localized and image-guided treatment.Int J Hyperthermia. 2020 Dec;37(3):141-154. doi: 10.1080/02656736.2020.1853252. Int J Hyperthermia. 2020. PMID: 33426994 Review.
-
Magnetic Particle Imaging-Guided Hyperthermia for Precise Treatment of Cancer: Review, Challenges, and Prospects.Mol Imaging Biol. 2023 Dec;25(6):1020-1033. doi: 10.1007/s11307-023-01856-z. Epub 2023 Oct 3. Mol Imaging Biol. 2023. PMID: 37789103 Review.
References
-
- Krishnan K. M., Fundamentals and Applications of Magnetic Materials, Oxford University Press, NY: 2016.
-
- Díez A. G., Rincón‐Iglesias M., Lanceros‐Méndez S., Reguera J., Lizundia E., Mater. Today Chem. 2022, 26, 101220.
-
- Colombo M., Carregal‐Romero S., Casula M. F., Gutiérrez L., Morales M. P., Böhm I. B., Heverhagen J. T., Prosperi D., Parak W. J., Chem. Soc. Rev. 2012, 41, 4306. - PubMed
-
- Gloag L., Mehdipour M., Chen D., Tilley R. D., Gooding J. J., Adv. Mater. 2019, 31, 1904385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
