Multimodal Artificial Intelligence-Based Virtual Biopsy for Diagnosing Abdominal Lavage Cytology-Positive Gastric Cancer
- PMID: 39985379
- PMCID: PMC12005817
- DOI: 10.1002/advs.202411490
Multimodal Artificial Intelligence-Based Virtual Biopsy for Diagnosing Abdominal Lavage Cytology-Positive Gastric Cancer
Abstract
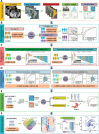
Gastric cancer with peritoneal dissemination remains a significant clinical challenge due to its poor prognosis and difficulty in early detection. This study introduces a multimodal artificial intelligence-based risk stratification assessment (RSA) model, integrating radiomic and clinical data to predict peritoneal lavage cytology-positive (GC-CY1) in gastric cancer patients. The RSA model is trained and validated across retrospective, external, and prospective cohorts. In the training cohort, the RSA model achieved an area under the curve (AUC) of 0.866, outperforming traditional clinical and radiomic feature models. External validation cohorts confirmed its robustness, with AUC values of 0.883 and 0.823 for predicting peritoneal metastasis and recurrence, respectively. In a prospective validation involving 152 patients, the model maintained superior predictive performance (AUC = 0.835). The RSA model also demonstrated significant clinical benefits by effectively identifying high-risk patients likely to benefit from specific treatments, such as paclitaxel-based conversion therapy. These findings suggest that the RSA model offers a reliable, non-invasive diagnostic tool for gastric cancer, capable of improving early detection and treatment outcomes. Further prospective studies are warranted to explore its full clinical potential.
Keywords: gastric cancer; multimodal artificial intelligence; peritoneal lavage cytology‐positive (CY1); radiomics; virtual biopsy.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R. L., Soerjomataram I., Jemal A., CA Cancer J. Clin. 2024, 74, 229. - PubMed
-
- Yamaguchi T., Takashima A., Nagashima K., Terashima M., Aizawa M., Ohashi M., Tanaka R., Yamada T., Kinoshita T., Matsushita H., Ishiyama K., Hosoda K., Yuasa Y., Haruta S., Kakihara N., Nishikawa K., Yunome G., Satoh T., Fukagawa T., Katai H., Boku N., Gastric Cancer 2021, 24, 701. - PubMed
-
- Yamaguchi T., Takashima A., Nagashima K., Makuuchi R., Aizawa M., Ohashi M., Tashiro K., Yamada T., Kinoshita T., Hata H., Kawachi Y., Kawabata R., Tsuji T., Hihara J., Sakamoto T., Fukagawa T., Katai H., Higuchi K., Boku N., Ann. Surg. Oncol. 2020, 27, 284. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
