Efficacy and Safety of Herbal Medicine Bojungikki-Tang in Combination with Pembrolizumab versus Pembrolizumab Monotherapy for Stage IV Non-Small Cell Lung Cancer: Study Protocol for a Randomized, Open-Label, Double-Arm, Multicenter Trial
- PMID: 39985390
- PMCID: PMC11847320
- DOI: 10.1177/15347354251319339
Efficacy and Safety of Herbal Medicine Bojungikki-Tang in Combination with Pembrolizumab versus Pembrolizumab Monotherapy for Stage IV Non-Small Cell Lung Cancer: Study Protocol for a Randomized, Open-Label, Double-Arm, Multicenter Trial
Abstract
Background: Non-small cell lung cancer (NSCLC) exhibits low survival rates. Although immune checkpoint inhibitors (ICIs) have become first-line treatment for NSCLC, their limited response to ICI monotherapy has led to exploration of combination treatments. However, the high incidence of treatment-related adverse events associated with conventional drug combinations has highlighted the need for alternative herbal therapy. Bojungikki-tang (BJIKT), a traditional herbal medicine, has been used to treat gastrointestinal disorders and enhance immune function. Our preclinical studies have demonstrated that BJIKT combined with anti-PD-1 or anti-PD-L1 antibodies exhibits significant efficacy in suppressing tumor growth by modulating the immunosuppressive tumor microenvironment. Building on these preclinical findings, this study aims to evaluate the efficacy and safety of BJIKT with pembrolizumab combination therapy compared to pembrolizumab monotherapy in advanced NSCLC patients.
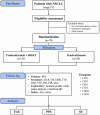
Methods: 70 individuals with stage IV NSCLC scheduled for first-line pembrolizumab monotherapy will be randomly assigned to intervention or control groups. The primary outcome will be progression-free survival, with secondary outcomes including disease control rate, overall survival, and quality of life assessment. Adverse events will be monitored for safety. This study will explore the synergistic mechanism of combinatorial therapy using immune profiling and multi-omics analysis, and the possibility for personalized integrative therapy based on cold-heat syndrome differentiation (SD) types in East Asian medicine.
Discussion: This study will provide novel evidence regarding survival outcomes, quality of life, and safety profiles of combined ICI and BJIKT therapy for advanced NSCLC. The exploratory data will contribute to tailoring treatments to immune-based SD types in NSCLC patients.
Keywords: Bojungikki-tang; non-small cell lung cancer; pembrolizumab; protocol; randomized controlled trial.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

