Role of tau versus TDP-43 pathology on medial temporal lobe atrophy in aging and Alzheimer's disease
- PMID: 39985478
- PMCID: PMC11846482
- DOI: 10.1002/alz.14582
Role of tau versus TDP-43 pathology on medial temporal lobe atrophy in aging and Alzheimer's disease
Abstract
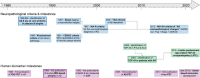
Hippocampal atrophy on magnetic resonance imaging is an important biomarker in Alzheimer's disease (AD). While hippocampal atrophy was thought to result from tau tangles in AD, different neuropathologies can lead to hippocampal atrophy, especially TAR DNA-binding protein 43 (TDP-43) pathology. In this narrative review, we evaluate existing studies on the relative contribution of tau and TDP-43 pathology to medial temporal lobe (MTL) atrophy. We report a clear association of both tau and TDP-43 neuropathology with MTL atrophy, even after correcting for other neuropathologies. Next, we discuss a potential synergism between tau and TDP-43 and the relative timing of the effects of both neuropathologies. Finally, avenues for future research will be discussed. A better understanding of the interplay between tau and TDP-43 neuropathologies and their effect on atrophy will help with the development of more specific biomarkers for limbic-predominant age-related TDP-43 encephalopathy and pinpointing of the optimal timing for testing anti-tau and anti-TDP-43 treatments in trials. HIGHLIGHTS: Both tau and TAR DNA-binding protein 43 (TDP-43) pathology contribute to medial temporal lobe atrophy. There is a positive association between tau and TDP-43 and potentially a synergism. It is unclear if tau and TDP-43 have an additive or synergistic effect on atrophy. The relative timing of the tau and TDP-43 effects on atrophy remains unclear. Clarifying the interplay between tau and TDP-43 will help improve magnetic resonance imaging biomarkers.
Keywords: Alzheimer's disease; TAR DNA‐binding protein 43; dementia; hippocampus; limbic‐predominant age‐related TDP‐43 encephalopathy; magnetic resonance imaging; medial temporal lobe; neurodegeneration; neurofibrillary tangle pathology.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Unrelated to the content of this manuscript, Dr. Jagust has served as a consultant to Lilly and has equity in Molecular Medicine and Optoceutics. None of the other authors have anything to disclose.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- R01-AG070592/AG/NIA NIH HHS/United States
- 2022-00900/Vetenskapsrådet
- MultiPark
- AF-980872/Alzheimerfonden
- U19-AG069701/AG/NIA NIH HHS/United States
- R01 AG070592/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- AARG-22-926899/ALZ/Alzheimer's Association/United States
- 20210690/Crafoordska Stiftelsen
- R01 AG073282/AG/NIA NIH HHS/United States
- AF-993465/Alzheimerfonden
- U19 AG069701/AG/NIA NIH HHS/United States
- P30-AG062422/AG/NIA NIH HHS/United States
- R01 AG062542/AG/NIA NIH HHS/United States
- R01-AG073282/AG/NIA NIH HHS/United States
- R01-AG062542/AG/NIA NIH HHS/United States

