Safety and Longevity of Intraocular Pressure Control After Bimatoprost Implant Administration: Interim Analysis of a Phase 3b Clinical Trial (TRITON)
- PMID: 39985740
- PMCID: PMC11947067
- DOI: 10.1007/s40265-025-02154-4
Safety and Longevity of Intraocular Pressure Control After Bimatoprost Implant Administration: Interim Analysis of a Phase 3b Clinical Trial (TRITON)
Abstract
Background: Bimatoprost implant 10 µg is an intracameral, biodegradable implant that slowly releases bimatoprost to lower intraocular pressure (IOP). This study was designed to evaluate safety and the duration of the IOP-lowering effect after single and as-needed repeat administration of the bimatoprost implant in patients with open-angle glaucoma (OAG) and ocular hypertension (OHT).
Patients and methods: This study is an interim analysis of an ongoing, prospective, open-label, multicenter study in patients with OAG or OHT who are inadequately managed with topical IOP-lowering medication for reasons other than efficacy. IOP-lowering rescue treatment is allowed if implant retreatment criteria are not met. The primary endpoint is time to retreatment/rescue after the initial implant administration analyzed with the Kaplan-Meier method. Key safety measures include treatment-emergent adverse events (TEAEs) and reading-center evaluation of central corneal endothelial cell density (CECD). Analysis of data collected through 15 September 2023 focused on outcomes after a single or two implants.
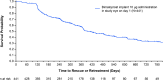
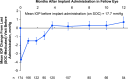
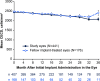
Results: In total, 441 patients received the 10-µg bimatoprost implant in the study eye on day 1 (cycle 1), 179 patients received a second administration (cycle 2), and 378 patients had at least 12 months of follow-up data available. The median time (95% confidence interval) from the first administration to a second administration or rescue was 392 (369, 485) days; the probability of not requiring retreatment or rescue by day 360 was 57.5%. A second implant administration similarly provided a long duration of IOP control. The baseline mean (standard error, SE) IOP was 25.6 (0.14) mmHg; the mean (SE) change from baseline IOP in unrescued eyes after a single administration was - 7.5 (0.21) mmHg at week 24 and - 6.4 (0.28) mmHg at month 12. Conjunctival hyperemia, typically associated with the administration procedure, was the most common ocular TEAE (cycle 1, 14.3%; cycle 2, 12.8%). Mean (SE) percentage change in CECD from baseline at 12 months after administration was - 4.3 (0.81)% in cycle 1 and - 8.5 (2.22)% in cycle 2. The cycle 1 implant was no longer visible or ≤ 25% of initial size in 66.3% and 94.3% of study eyes at months 12 and 24, respectively.
Conclusions: In this interim analysis based on available data, the IOP-lowering effect of the initial administration of the 10-µg bimatoprost implant was well maintained for > 1 year in most patients. Results after a second administration were comparable. The safety profile of initial and repeat administration was acceptable.
Trial registry: ClinicalTrials.gov identifier NCT03850782; registered 20 February 2019.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Allergan (prior to its acquisition by AbbVie) and/or AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. Conflict of interest: Amanda K. Bicket is a consultant for W.L. Gore and Associates, Inc. Christian Brinkmann has received financial compensation from AbbVie, Afidera, Appelis, Bayer, Heidelberg Engineering, Novartis, Santen, Teleon Surgical, and Théa Laboratories. William C. Christie is a consultant for Allergan (an AbbVie company). Petrus N.J. Gous has no financial interests to disclose. Miriam Kolko is a speaker for AbbVie, Santen, Théa Laboratories, and Topcon; and sits on advisory boards for AbbVie, Santen, and Théa Laboratories. Jan Luebke is a speaker for AbbVie, Alcon, Glaukos, iStar Medical, Santen, and Théa Laboratories and sits on advisory boards for AbbVie and Santen. Francesco Oddone is a speaker for AbbVie, Omikron Italia, Santen, Sifi, and Theà; sits on advisory boards for AbbVie, Dompè, and Santen; and has received research grants from AbbVie, Santen, and Omikron Italia. Steven M. Silverstein has no disclosures beyond participation in this clinical study. Marina Bejanian, E. Randy Craven, Jenny Jiao, Jyotsna Maram, Ashley Nguyen, Yongjia Pu, and Michael R. Robinson are employees of AbbVie and may hold AbbVie stock. Ethics approval: Institutional review board or independent ethics committee approval was obtained at each site before the study began, and the study was performed in compliance with Good Clinical Practice, the principles of the Declaration of Helsinki, and applicable laws and regulations (ClinicalTrials.gov registration number NCT03850782). Consent for participation: All patients in this study provided written informed consent before undergoing any study-related procedure. Data availability: AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research and will be provided following review and approval of a research proposal, statistical analysis plan (SAP), and execution of a data sharing agreement (DSA). Data requests can be submitted at any time after approval in the USA and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/ . Code availability: Not applicable. Author contributions: Study design: M.B. and A.N.; data collection: A.K.B., C.K.B., W.C.C., P.N.J.G., J.L., M.K., F.O., and S.M.S.; statistical analysis: J.J. and Y.P.; data interpretation: M.B., A.K.B., C.K.B., W.C.C., P.N.J.G., J.J., J.L., M.K., J.M., A.N., F.O., Y.P., and S.M.S.; drafting, revision, and approval of the manuscript: M.B., A.K.B., C.K.B., W.C.C., P.N.J.G., J.J., J.L., M.K., J.M., A.N., F.O., Y.P., and S.M.S.
Figures


References
-
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. 10.1016/j.ophtha.2014.05.013. - PubMed
-
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. 10.1001/archopht.121.1.48. - PubMed
-
- Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–304. 10.1016/s0140-6736(14)62111-5. - PubMed
-
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. (discussion 829-30). 10.1001/archopht.120.6.701. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

