Adverse prognosis gene expression patterns in metastatic castration-resistant prostate cancer
- PMID: 39985777
- PMCID: PMC12330944
- DOI: 10.1002/1878-0261.70001
Adverse prognosis gene expression patterns in metastatic castration-resistant prostate cancer
Abstract
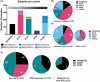
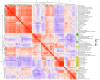
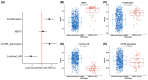
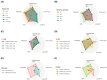
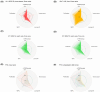
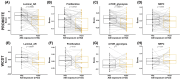
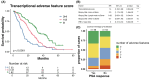
Metastatic castration-resistant prostate cancer (mCRPC) is a heterogeneous disease. Several studies have identified transcriptional subtypes of mCRPC, but comprehensive analysis of prognostic gene expression pathways has been limited. Therefore, we aggregated a cohort of 1012 mCRPC tissue samples from 769 patients and investigated the association of gene expression-based pathways with clinical outcomes and intrapatient and intratumor heterogeneity. Survival data were obtained for 272 patients. Pathway-level enrichment was evaluated using gene set variation analysis. scRNA-seq datasets from mCRPC tissue biopsies and circulating tumor cells were used to investigate heterogeneity of adverse pathways. We identified five pathway clusters: (a) Immune response/WNT/TGF-beta signaling, (b) AR signaling/luminal signatures, (c) mTOR signaling and glycolysis, (d) cell proliferation, and (e) neuroendocrine differentiation. Proliferation, AR signaling loss, and glycolysis/mTOR signaling were independently prognostic. Adverse prognostic pathway scores decreased on treatment with AR signaling inhibitors, but not at progression, suggesting failure to permanently target these pathways. scRNA-seq datasets from mCRPC tissue biopsies and circulating tumor cells were used to investigate heterogeneity of adverse pathways. Our results suggest loss of AR signaling, high proliferation, and a glycolytic phenotype as adverse prognostic pathways in mCRPC that could be used in conjunction with clinical factors to prognosticate for treatment decisions.
Keywords: biomarker; gene expression; metastatic castration‐resistant prostate cancer; precision medicine; prognosis.
© 2025 The Author(s). Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
KTH has a family member who is an employee of Epic Systems. YS reports employment at Tempus with restricted stock units. MB has a family member who is an employee of Luminex. SGZ reports unrelated patents licensed to Veracyte, and that a family member is an employee of Artera and holds stock in Exact Sciences. SMD reports consulting relationships with BMS, Oncternal therapeutics, Janssen R&D/J&J and a grant from Pfizer/Astellas/Medivation (the grant was submitted to Medivation, ultimately funded by Astellas and then moved to Pfizer). EJS reports honoraria from Janssen for serving on Advisory Board and honoraria and stock options from Fortis Therapeutics. MNS reports institutional research support from Novartis. MS reports speaker fees from Astellas and consulting fees for serving on Advisory Board from Veracyte/Adelphi Targis.
Figures
References
MeSH terms
Grants and funding
- R35 GM142441/GM/NIGMS NIH HHS/United States
- University of Wisconsin Office of the Vice Chancellor
- 1UH2CA260389/NH/NIH HHS/United States
- R01CA270539/NH/NIH HHS/United States
- R01CA174777/NH/NIH HHS/United States
- Prostatacancerförbundet
- R01CA259388/NH/NIH HHS/United States
- HT94252410252/Department of Defense
- Knut och Alice Wallenbergs Stiftelse
- P50 CA275741/CA/NCI NIH HHS/United States
- R01 CA270539/CA/NCI NIH HHS/United States
- R01 CA276269/CA/NCI NIH HHS/United States
- R50 CA293840/CA/NCI NIH HHS/United States
- PC200334/Department of Defense
- R01 CA174777/CA/NCI NIH HHS/United States
- Benioff Initiative for Prostate Cancer Research
- 2021088/DDCF/Doris Duke Charitable Foundation/United States
- PC220240/Department of Defense
- Junior Clinical Investigator Award/Cancerfonden
- 1P50CA275741/NH/NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- R01 CA259388/CA/NCI NIH HHS/United States
- R01 CA247479/CA/NCI NIH HHS/United States
- PC180469/Department of Defense
- Hjelms Stiftelse för medicinsk forskning
- UH2 CA260389/CA/NCI NIH HHS/United States
- R01CA247479/NH/NIH HHS/United States
- DP2 OD030734/NH/NIH HHS/United States
- R35GM142441/NH/NIH HHS/United States
- PC190039/Department of Defense
- P30 CA014520/NH/NIH HHS/United States
- W81XWH-22-1-0833/Department of Defense
- Prostate Cancer Foundation
- R01CA276269/NH/NIH HHS/United States
- 21 1856 Pj/Cancerfonden
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

