Experience in Ceftazidime-Avibactam for treatment of MDR BGN infection in Oncologic Children
- PMID: 39985933
- PMCID: PMC11893299
- DOI: 10.1016/j.bjid.2025.104515
Experience in Ceftazidime-Avibactam for treatment of MDR BGN infection in Oncologic Children
Abstract
Background: Ceftazidime-Avibactam (CAZ-AVI) plays a key role in the treatment of Multidrug Resistant Gram-Negative Bacilli (MDR-GNB) infections. In pediatrics, CAZ-AVI is clinically approved for treatment of urinary tract or intra-abdominal infection. However, there is limited data available about its use in children with cancer who have complicated infections caused by MDR-GNB.
Objective: This study aims to describe our experience in using CAZ-AVI for the treatment of MDR GNB infections in children with cancer.
Methods: This retrospective observational study was conducted at the Pediatric Oncology Institute (IOP/GRAACC/UNIFESP), including pediatric oncologic patients who received CAZ-AVI for the treatment of infections caused by GNB.
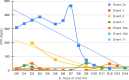
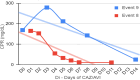
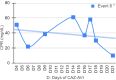
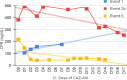
Results: From Jan/2021 to Jun/2022, 11 patients with 13 episodes were included in the analysis. Among them, 45 % were female, with a median age of 7 years. Three patients had Acute lymphoblastic Leukemia (ALL), three had Acute Myeloid Leukemia (AML), two had Non-Hodgkin Lymphoma (NHL). Additionally, there was one case each of medulloblastoma, fibrosarcoma, and craniopharyngioma. All patients presented significant risk factors for MDR-GNB, such as neutropenia and two were submitted to Hematopoietic Stem Cell Transplantation (HSCT). The infection episodes included six Bloodstream Infections (BSI), two Urinary Tract Infections (UTI), two tracheobronchitis cases, along with one case each of necrotizing pneumonia, ventriculitis, and endocarditis. The identified pathogens included Klebsiella pneumoniae, Pseudomonas spp., Enterobacter cloacae, and Stenotrophomonas maltophilia. The primary reason for prescribing CAZ-AVI was either Multidrug-Resistant Gram-Negative Bacteria (MDR-GNB) infection or clinical worsening after initial therapy. Combination therapy was prescribed in eight episodes with a median prescription length of nine days. Microbiological sterilization was achieved in 92 % of episodes, and the 30-day survival rate was 84 %. Notably, no deaths were associated with treatment failure, and no adverse events associated with CAZ-AVI use were observed.
Conclusion: CAZ-AVI could be used for treating GNB infections in oncologic pediatric patients.
Keywords: Cancer; Ceftazidime-avibactam; Children; Febrile neutropenia; Gram negative bacilli infection; Multiresistance; Oncology; Pediatric.
Copyright © 2025. Published by Elsevier España, S.L.U.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest.
Figures
References
-
- Zajac-Spychala O, Wachowiak J, Gryniewicz-Kwiatkowska O, Gietka A, Dembowska-Baginska B, Semczuk K, et al. Prevalence, epidemiology, etiology, and sensitivity of invasive bacterial infections in pediatric patients undergoing oncological treatment: A multicenter nationwide study. Microb Drug Resist. 2021;27:53–63. - PubMed
-
- Santolaya ME, Alvarez AM, Becker A, Cofré J, Enríquez N, O'Ryan M, et al. Prospective, multicenter evaluation of risk factors asalsociated with invasive bacterial infection in children with cancer, neutropenia, and fever. J Clin Oncol. 2001;19:3415–3421. - PubMed
-
- Schimpff S, Satterlee W, Young VM, Serpick A. Empiric therapy with Carbenicillin and Gentamicin for Febrile patients with cancer and granulocytopenia. New Engl J Med. 1971;284:1061–1065. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous