Autoinflammatory encephalopathy due to PTPN1 haploinsufficiency: a case series
- PMID: 39986310
- PMCID: PMC7617446
- DOI: 10.1016/S1474-4422(24)00526-X
Autoinflammatory encephalopathy due to PTPN1 haploinsufficiency: a case series
Abstract
Background: Through the agnostic screening of patients with uncharacterised disease phenotypes for an upregulation of type I interferon (IFN) signalling, we identified a cohort of individuals heterozygous for mutations in PTPN1, encoding the protein-tyrosine phosphatase 1B (PTP1B). We aimed to describe the clinical phenotype and molecular and cellular pathology of this new disease.
Methods: In this case series, we identified patients and collected clinical and neuroradiological data through collaboration with paediatric neurology and clinical genetics colleagues across Europe (Czechia, France, Germany, Italy, Slovenia, and the UK) and Israel. Variants in PTPN1 were identified by exome and directed Sanger sequencing. The expression of IFN-stimulated genes was determined by quantitative (q) PCR or NanoString technology. Experiments to assess RNA and protein expression and to investigate type 1 IFN signalling were undertaken in patient fibroblasts, hTERT-immortalised BJ-5ta fibroblasts, and RPE-1 cells using CRISPR-Cas9 editing and standard cell biology techniques.
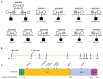
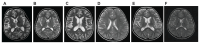
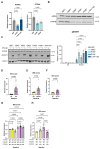
Findings: Between Dec 20, 2013, and Jan 11, 2023, we identified 12 patients from 11 families who were heterozygous for mutations in PTPN1. We found ten novel or very rare variants in PTPN1 (frequency on gnomAD version 4.1.0 of <1·25 × 10:sup>-6). Six variants were predicted as STOP mutations, two involved canonical splice-site nucleotides, and two were missense substitutions. In three patients, the variant occurred de novo, whereas in nine affected individuals, the variant was inherited from an asymptomatic parent. The clinical phenotype was characterised by the subacute onset (age range 1-8 years) of loss of motor and language skills in the absence of seizures after initially normal development, leading to spastic dystonia and bulbar involvement. Neuroimaging variably demonstrated cerebral atrophy (sometimes unilateral initially) or high T2 white matter signal. Neopterin in CSF was elevated in all ten patients who were tested, and all probands demonstrated an upregulation of IFN-stimulated genes in whole blood. Although clinical stabilisation and neuroradiological improvement was seen in both treated and untreated patients, in six of eight treated patients, high-dose corticosteroids were judged clinically to result in an improvement in neurological status. Of the four asymptomatic parents tested, IFN signalling in blood was normal (three patients) or minimally elevated (one patient). Analysis of patient blood and fibroblasts showed that tested PTPN1 variants led to reduced levels of PTPN1 mRNA and PTP1B protein, and in-vitro assays demonstrated that loss of PTP1B function was associated with impaired negative regulation of type 1 IFN signalling.
Interpretation: PTPN1 haploinsufficiency causes a type 1 IFN-driven autoinflammatory encephalopathy. Notably, some patients demonstrated stabilisation, and even recovery, of neurological function in the absence of treatment, whereas in others, the disease appeared to be responsive to immune suppression. Prospective studies are needed to investigate the safety and efficacy of specific immune suppression approaches in this disease population.
Funding: The UK Medical Research Council, the European Research Council, and the Agence Nationale de la Recherche.
Copyright © 2025 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests TG reports a grant for the impact of anti-viral immunity in solid organ transplantation: mechanisms of host infection control in immunosuppressed and infection-prone individuals (TTU07.822_00) from Deutsches Zentrum für Infektionsforschung. OBT receives payment from HCERES (the French Scientific Authority), HAS (the French Health Authority), Italfarmaco, and Minoryx for expert testimony. OBT serves on the Data and Safety Monitoring Board for Minoryx and as the unpaid Scientific Council President of AFM Telethon. YH receives grants from Great Ormond Street Hospital (GOSH) Children Charity and MS Society UK, honoraria from the AAN Continuum, and has served unpaid roles in the Neurology Journal Editorial Board, the MS Society UK Advisory Group for Medications, the MOG Project Medical Advisory Board, and the Guthy-Jackson Foundation International Clinical Consortium. ML has received the following grants: the minimal motion system for MRI: MR-MinMo (Clinical Lead) from the National Institute for Health and Care Research (NIHR204201); multimodal assessment of remyelination and light-chain neurofilament assay following demyelination episodes in children (MARMALADE-C; Doctoral supervision) from Action Medical Research (GN2945); investigating the neurological impact of COVID-19 in non-hospitalised children with persistent symptoms (Clinical Lead) from Action Medical Research (GN2925); validation of the paediatric autoimmune encephalitis severity score (PASS) in children with autoimmune encephalitis (clinical supervision) from the Encephalitis Society; prognosis, treatment, and mechanisms in an international paediatric-onset opsoclonus myoclonus ataxia syndrome study (POOMAS; UK Lead) from the Boston Children's Hospital Research Fund (GENFD0001772273); developing magnetic resonance measures of neurobiological dysfunction in early recovery from NMDAR-antibody encephalitis (Doctoral Supervisor) from Action Medical Research (GN2835); and long-term sequelae following PIM-TS (study co-lead) from GOSH Charity Rapid Response Funding Call (VC1421). ML has received consulting fees from Roche, Novartis, and Octapharma as part of the respective Expert Advisory Board. ML gives around four lectures, presentations, and meetings per year and all honoraria are paid to ML's institutional research account. ML performs up to six medical legal cases a year, and serves as data monitoring committee Chair in the AGSRTI Trial evaluating reverse transcriptase inhibition as treatment in Aicardi-Goutières syndrome (NCT04731103). ML serves as on the steering committee in the phase III study to compare the effect of panzyga versus placebo in patients with paediatric acute-onset neuropsychiatric syndrome (NCT04508530), as unpaid co-Chair of the European Paediatric Neurology Society Education and Training Board, as unpaid co-Chair of the James Lind Alliance and British Paediatric Neurology Association Research Priority Setting Partnership, and has a paid leadership role as Associate Editor of the European Journal of Paediatric Neurology. MMM receives payment from Jazz Pharmaceuticals and serves on the Advisory Board of Italfarmaco. IM receives payments from Boehringer Ingelheim and GSK, and support for travel and meetings from Novartis. All other authors declare no competing interests.
Figures

References
-
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7(11):833–46. - PubMed
-
- Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544–8. - PubMed
-
- Gunawardana J, Chan FC, Telenius A, et al. Recurrent somatic mutations of PTPN1 in primary mediastinal B cell lymphoma and Hodgkin lymphoma. Nat Genet. 2014;46(4):329–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

