Amyotrophic lateral sclerosis caused by hexanucleotide repeat expansions in C9orf72: from genetics to therapeutics
- PMID: 39986312
- PMCID: PMC12010636
- DOI: 10.1016/S1474-4422(25)00026-2
Amyotrophic lateral sclerosis caused by hexanucleotide repeat expansions in C9orf72: from genetics to therapeutics
Abstract
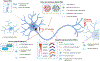
GGGGCC repeat expansions in C9orf72 are a common genetic cause of amyotrophic lateral sclerosis in people of European ancestry; however, substantial variability in the penetrance of the mutation, age at disease onset, and clinical presentation can complicate diagnosis and prognosis. The repeat expansion is bidirectionally transcribed in the sense and antisense directions into repetitive RNAs and translated into dipeptide repeat proteins, and both accumulate in the cortex, cerebellum, and the spinal cord. Furthermore, neuropathological aggregates of phosphorylated TDP-43 are observed in motor cortex and other cortical regions, and in the spinal cord of patients at autopsy. C9orf72 repeat expansions can also cause frontotemporal dementia. The GGGGCC repeat induces a complex interplay of loss-of-function and gain-of-function pathological mechanisms. Clinical trials using antisense oligonucleotides to target the GGGGCC repeat RNA have not been successful, potentially because they only target a single gain-of-function mechanism. Novel therapeutic approaches targeting the DNA repeat expansion, multiple repeat-derived RNA species, or downstream targets of TDP-43 dysfunction are, however, on the horizon, together with the development of diagnostic and prognostic biomarkers.
Copyright © 2025 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests SM received payments to her institution from UK Dementia Research Institute, the Motor Neurone Disease (MND) Association, the Packard Centre for ALS Research, Van Geest Neurosciences Donation, Alzheimer's Research UK, and ONO Pharmaceuticals; and participates in Discovery Network Advisory Board: My Name5 Doddie. TFG received funding from the National Institutes of Health, National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS; P30 AG062677, U19 AG063911, P01 NS084974, R01 NS117461, and R01 NS121125); and received inventor intellectual property royalties from Ionis Pharmaceuticals, Takeda, Biogen, and Jackson Laboratory, and the Target ALS for her involvement in the development of the C9orf72 repeat expansion construct and an AAV-C9orf72 repeat expansion mouse model. GMH received grants from the Medical Research Council (MRC; grants MR/W00416X/1 and MR/Z506229/1), the Biotechnology and Biological Sciences Research Council (grant BB/S005277/1), and the LifeArc Philanthropic Fund MND Association grant 878-791; and is the Founding Director of Crucible Therapeutics and the primary inventor of granted and pending patents related to the use of SRSF1 inhibitors to treat neurological disorders, including C9orf72-ALS (C9orf72-associated amyotrophic lateral sclerosis) or C9orf72-FTD (C9orf72-associated frontotemporal dementia). OH received grant support from the Science Foundation Ireland and Health Research Board; consulting fees from Biogen and Wave Pharmaceuticals; performs editorial duties for Taylor and Francis; and served on the data safety board for MediNova and advisory board for Novartis. AMI received funding from the UK Dementia Research Institute, principally funded by the MRC, and additional funding partners LifeArc and ARUK; and is a co-inventor on UK (2105455.6) and international patents (PCT/EP2022/060296) for “CasRx/Cas13d systems targeting C9orf72” to target both sense and antisense C9orf72 repeats. MvB receives funding from NINDS (RF1 NS123052 and R01 NS121125) and the Spastic Paraplegia Foundation. RR received funding from NIA and the NINDS (U19 AG063911 and UG3 NS103870), the US Department of Defense, The Fund Generet, and the Fund for Scientific Research Flanders; and is an author on a patent entitled: “Detecting Frontotemporal Dementia and Amyotrophic Lateral sclerosis” (US 14343807). JR participated in clinical trials of ASO for C9orf72-ALS sponsored by Biogen Pharmaceuticals; is on the scientific advisory board of Transposon; and holds USA patents on methods for modulating C9or72 expression and antisense expression. RR declares no competing interests.
Figures

References
-
- Viodé A, Fournier C, Camuzat A, Fenaille F, Latouche M, Elahi F, et al. New Antibody-Free Mass Spectrometry-Based Quantification Reveals That C9ORF72 Long Protein Isoform Is Reduced in the Frontal Cortex of Hexanucleotide-Repeat Expansion Carriers. Front Neurosci. 2018. -August-28;12. - PMC - PubMed

