Prolonged versus single dose in penicillin oral challenge testing: protocols for a pilot and definitive randomised controlled trial (PROSPECTOR studies)
- PMID: 39987001
- PMCID: PMC11848670
- DOI: 10.1136/bmjopen-2024-094712
Prolonged versus single dose in penicillin oral challenge testing: protocols for a pilot and definitive randomised controlled trial (PROSPECTOR studies)
Abstract
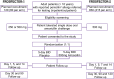
Introduction: Penicillin allergy labels (PALs) are reported in 1 in 10 hospitalised patients globally and associated with inferior patient, hospital and microbiological outcomes; however, the majority are incorrect and should be removed. Direct oral penicillin challenge has been demonstrated to be a safe and effective method for the removal of PALs. However, the question of whether a single dose is sufficient to ascertain true allergy status remains unanswered, with some studies suggesting that extended challenges of 3 or more days are superior for the exclusion of delayed immune reactions. The aim of the PROSPECTOR studies was to determine the feasibility (PROSPECTOR-1) of a definitive trial (PROSPECTOR-2) to evaluate the safety and effectiveness of prolonged oral challenge (ie, 5 days) versus single-dose oral challenge in patients with a delayed or unknown penicillin allergy phenotype (PROSPECTOR-2).
Methods and analysis: A pair of double-blind two-arm parallel placebo-controlled trials will be undertaken-PROlonged versus Single dose in PEnicillin oral Challenge Testing double-blind parallel group randomised placebo-cOntrolled tRial (PROSPECTOR Studies). Patients with a reported delayed or unknown timing penicillin allergy who have passed a supervised single-dose oral amoxicillin challenge (with or without prior skin testing/single or split dose) will be recruited. Informed patient consent will be granted for sites to recruit patients and collect routine clinical data. PROSPECTOR-1 will assess the safety and feasibility of a placebo-controlled trial for single-dose amoxicillin challenge versus 5-day prolonged oral challenge. PROSPECTOR-2 will assess the superiority of the 5-day prolonged oral challenge compared with single-dose amoxicillin challenge in excluding a delayed immune reaction. PROSPECTOR-2 will commence immediately post completion of PROSPECTOR-1 in a vanguard design, with adjustments to the projected sample size for superiority made following completion of PROSPECTOR-1. PROSPECTOR-2 will commence recruitment immediately following closure of PROSPECTOR-1; however, data from each trial will be analysed separately.
Ethics and dissemination: These studies were reviewed and approved by the Austin Health Human Research Ethics Committee (PROSPECTOR-1: HREC/99740/Austin-2023 and PROSPECTOR-2: HREC/109785/Austin-2024). The results will be published in peer-reviewed journals and presented at relevant conferences.
Trial registration number: PROSPECTOR-1: ACTRN12623001242617 and PROSPECTOR-2: ACTRN12624001107516.
Keywords: Antibiotics; Clinical Trial; IMMUNOLOGY; PUBLIC HEALTH; Protocols & guidelines.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
