YY1 mutations disrupt corticogenesis through a cell type specific rewiring of cell-autonomous and non-cell-autonomous transcriptional programs
- PMID: 39987231
- PMCID: PMC12240807
- DOI: 10.1038/s41380-025-02929-x
YY1 mutations disrupt corticogenesis through a cell type specific rewiring of cell-autonomous and non-cell-autonomous transcriptional programs
Abstract
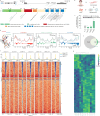
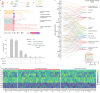
Germline mutations of YY1 cause Gabriele-de Vries syndrome (GADEVS), a neurodevelopmental disorder featuring intellectual disability and a wide range of systemic manifestations. To dissect the cellular and molecular mechanisms underlying GADEVS, we combined large-scale imaging, single-cell multiomics and gene regulatory network reconstruction in 2D and 3D patient-derived physiopathologically relevant cell lineages. YY1 haploinsufficiency causes a pervasive alteration of cell type specific transcriptional networks, disrupting corticogenesis at the level of neural progenitors and terminally differentiated neurons, including cytoarchitectural defects reminiscent of GADEVS clinical features. Transcriptional alterations in neurons propagated to neighboring astrocytes through a major non-cell autonomous pro-inflammatory effect that grounds the rationale for modulatory interventions. Together, neurodevelopmental trajectories, synaptic formation and neuronal-astrocyte cross talk emerged as salient domains of YY1 dosage-dependent vulnerability. Mechanistically, cell type resolved reconstruction of gene regulatory networks uncovered the regulatory interplay between YY1, NEUROG2 and ETV5 and its aberrant rewiring in GADEVS. Our findings underscore the reach of advanced in vitro models in capturing developmental antecedents of clinical features and exposing their underlying mechanisms to guide the search for targeted interventions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All methods were performed in accordance with the relevant guidelines and regulations. This study was approved by the institutional review board of the University of Milan ethics committee. Written informed consents were obtained for all individuals. All animal experiments were done in accordance with the Italian Laws (D.L.vo 116/92 and following additions), which enforces EU 86/609 Directive (Council Directive 86/609/EEC of 24 November 1986), and were approved by institutional ethics committee (organismo preposto al benessere degli animali, OPBA committee) and the Italian Ministry of Health (Authorization 1073/16-PR).
Figures





Update of
-
YY1 mutations disrupt corticogenesis through a cell-type specific rewiring of cell-autonomous and non-cell-autonomous transcriptional programs.bioRxiv [Preprint]. 2024 Feb 17:2024.02.16.580337. doi: 10.1101/2024.02.16.580337. bioRxiv. 2024. Update in: Mol Psychiatry. 2025 Aug;30(8):3413-3429. doi: 10.1038/s41380-025-02929-x. PMID: 38405909 Free PMC article. Updated. Preprint.
References
-
- Vissers LELM, de Ligt J, Gilissen C, Janssen I, Steehouwer M, de Vries P, et al. A de novo paradigm for mental retardation. Nat Genet. 2010;42:1109–12. - PubMed
-
- Ferng A, Thulin P, Walsh E, Weissbrod PA, Friedman J. YY1: a new gene for childhood onset dystonia with prominent oromandibular-laryngeal involvement? Mov Disord Off J Mov Disord Soc. 2022;37:227–8. - PubMed
-
- Zorzi G, Keller Sarmiento IJ, Danti FR, Bustos BI, Invernizzi F, Panteghini C, et al. YY1-related dystonia: clinical aspects and long-term response to deep brain stimulation. Mov Disord Off J Mov Disord Soc. 2021;36:1461–2. - PubMed
-
- Nabais Sá MJ, Gabriele M, Testa G, de Vries BB. Gabriele-de Vries Syndrome. GeneReviews®. Seattle (WA): University of Washington, Seattle; 2019. - PubMed
MeSH terms
Substances
Grants and funding
- Innovative Training Network EpiSyStem/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 765966/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)

