Excess of rare noncoding variants in several type 2 diabetes candidate genes among Asian Indian families
- PMID: 39987249
- PMCID: PMC11846969
- DOI: 10.1038/s43856-025-00750-9
Excess of rare noncoding variants in several type 2 diabetes candidate genes among Asian Indian families
Abstract
Background: Type 2 diabetes (T2D) etiology is highly complex due to its multiple roots of origin. Polygenic risk scores (PRS) based on genome-wide association studies (GWAS) can partially explain T2D risk. Asian Indian people have up to six times higher risk of developing T2D than European people, and underlying causes of this disparity are unknown.
Methods: We have performed targeted sequencing of ten T2D GWAS/candidate regions using endogamous Punjabi Sikh families and replication studies using unrelated Sikh people and families from three other Indian endogamous ethnic groups (EEGs).
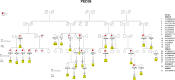
Results: We detect rare and ultra-rare variants (RVs) in KCNJ11-ABCC8 and HNF4A (MODY genes) cosegregated with late-onset T2D. We also identify RV enrichment in two new genes, SLC38A11 and ANPEP, associated with T2D. Gene-burden analysis reveals the highest RV burden contributed by HNF4A (p = 0.0003), followed by KCNJ11/ABCC8 (p = 0.0061) and SLC38A11 (p = 0.03). Some RVs detected in Sikh people are also found in Agarwals from Jaipur, both from Northern India, but were monomorphic in other two EEGs from South Indian people. Despite carrying a high burden of T2D and RVs, most families have a significantly lower burden of PRS. Functional studies show that an intronic regulatory variant (RV) in ABCC8 affects the binding of Pax4 and NF-kB transcription factors, influencing downstream gene regulation.
Conclusions: The high burden of T2D in these families may stem from the enrichment of noncoding RVs in a small number of major known genes (including MODY genes) with oligogenic inheritance alongside RVs from genes associated with polygenic susceptibility. These findings highlight the need to conduct deeper evaluations of families from non-European ancestries to identify potential novel therapeutics and implement preventative strategies.
Plain language summary
People with type 2 diabetes (T2D) have high levels of sugar in the blood, which can cause many health problems. T2D is a major global health issue, with Asian Indian people being up to six times more likely to develop it than European people. Although inherited factors contribute to this increased risk, they only partially explain the high T2D prevalence in Asian Indian families. In our study, we sequenced known T2D-related genes in Punjabi Sikh families. We found rare changes in inherited genes known to increase the incidence of T2D. Our findings emphasize the importance of investigating families from non-European backgrounds to identify new treatment and prevention strategies for T2D.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- L’Heveder, R. & Nolan, T. International Diabetes Federation. Diab. Res. Clin. Pract.101, 349–351 (2013). - PubMed
-
- Chen, L., Magliano, D. J. & Zimmet, P. Z. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat. Rev. Endocrinol.8, 228–236 (2011). - PubMed
-
- McKeigue, P. M., Pierpoint, T., Ferrie, J. E. & Marmot, M. G. Relationship of glucose intolerance and hyperinsulinaemia to body fat pattern in south Asians and Europeans. Diabetologia35, 785–791 (1992). - PubMed
-
- Ogurtsova, K. et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diab. Res. Clin. Pract.128, 40–50 (2017). - PubMed
Grants and funding
- R01DK082766, R01DK118427/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- 55/6/2/Indo-US/ 2014-NCD-II/Indian Council of Medical Research (ICMR)
- R21DK105913/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- R01 DK118427/DK/NIDDK NIH HHS/United States
- R01 DK082766/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

