EZH2-mediated macrophage-to-myofibroblast transition contributes to calcium oxalate crystal-induced kidney fibrosis
- PMID: 39987296
- PMCID: PMC11846861
- DOI: 10.1038/s42003-025-07735-3
EZH2-mediated macrophage-to-myofibroblast transition contributes to calcium oxalate crystal-induced kidney fibrosis
Abstract
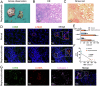
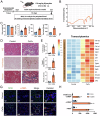
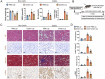
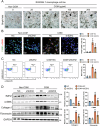
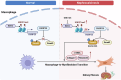
Long-term nephrocalcinosis leads to kidney injury, fibrosis, and even chronic kidney disease (CKD). Macrophage-to-myofibroblast transition (MMT) has been identified as a new mechanism in CKD, however, the effect of MMT in calcium oxalate (CaOx)-induced kidney fibrosis remains unclear. In this study, abundant MMT cells are identified by immunofluorescence (IF) and flow cytometry in kidney tissues of patients with CaOx-related CKD, a male mouse model, and CaOx-treated macrophages. Clodronate liposome (CLO)-mediated macrophage depletion attenuates fibrosis in male nephrocalcinosis mice. Transcriptomic sequencing reveals that histone methyltransferase (HMTs), EZH2, is highly expressed in nephrocalcinosis. Ezh2 inducible knock-out or inhibition by GSK-126 attenuates MMT and renal fibrosis. Mechanistically, ChIP and transcriptomic sequencing show that EZH2 inhibition reduces the enrichment of H3K27me3 on the Dusp23 gene promoter and elevates Dusp23 expression. The Co-IP and molecular docking analysis shows that DUSP23 mediates the dephosphorylation of pSMAD3 (Ser423/425). Thus, our study found that EZH2 promotes kidney fibrosis by meditating MMT via the DUSP23/SMAD3 pathway in nephrocalcinosis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Zeng, G. et al. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int.120, 109–116 (2017). - PubMed
-
- Thongprayoon, C., Krambeck, A. E. & Rule, A. D. Determining the true burden of kidney stone disease. Nat. Rev. Nephrol.16, 736–746 (2020). - PubMed
-
- Khan, S. R., Canales, B. K. & Dominguez-Gutierrez, P. R. Randall’s plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat. Rev. Nephrol.17, 417–433 (2021). - PubMed
MeSH terms
Substances
Grants and funding
- 82370765/National Natural Science Foundation of China (National Science Foundation of China)
- 82170775/National Natural Science Foundation of China (National Science Foundation of China)
- 82400895/National Natural Science Foundation of China (National Science Foundation of China)
- 82270802/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

