Patient Experience of Treatment with Tirzepatide for Weight Management: Exit Interviews from SURMOUNT-4
- PMID: 39987302
- PMCID: PMC11985590
- DOI: 10.1007/s40271-025-00730-0
Patient Experience of Treatment with Tirzepatide for Weight Management: Exit Interviews from SURMOUNT-4
Abstract
Background and objectives: Tirzepatide is a glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist, which was approved in 2023 by the US Food and Drug Administration for weight management in adults with obesity or overweight. The purpose of this study was to conduct qualitative exit interviews with participants who had participated in the SURMOUNT-4 clinical trial, to better understand the patient experience of tirzepatide.
Methods: Online exit interviews were conducted with adults from the USA who had participated in the SURMOUNT-4 clinical trial for weight management, recruited from 16 US-based SURMOUNT-4 clinical sites. Interviews utilized a semi-structured interview guide, and included questions related to receiving tirzepatide, using a single-use injection pen device, and the overall trial experience. Interviews were audio recorded and transcribed, and analyzed using a content analysis.
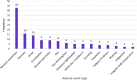
Results: Eighty-six adults (83% female; mean age 49.9 years) participated in the interviews. All participants shared at least one perceived benefit of tirzepatide experienced during the open-label phase of SURMOUNT-4, including improved appetite control, increased energy, or improved clothing fit. Despite the gastrointestinal side effects experienced, many participants liked the efficacy of tirzepatide, and reported that the single-use injection pen device for administering the study medication was easy to use. Most participants were willing to continue taking tirzepatide.
Conclusions: Study findings showed that beyond the direct pharmacological effects of treatment with tirzepatide, participants reported a wide range of perceived improvements across several aspects of their lives. Participants also reported a few negative experiences, including side effects. It is possible that the participants who had a more positive experience were more inclined to participate in the exit interviews. This study highlights the value of exit interviews, which can provide more learning about patient experiences during a clinical trial.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This study was funded by Eli Lilly and Company. Conflict of interest: Chloe Carmichael, Elizabeth Collins, Danielle Burns, and Helen Kitchen are employed by Clarivate, a company that received funding from Eli Lilly and Company for time spent conducting this research. Irina Jouravskaya, Jiat Ling Poon, Donna Mojdami, Madhumita Murphy, Nadia Ahmad, and Chisom Kanu are employed by and own stock in Eli Lilly and Company. Ethics approval: This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study protocol and associated documents were submitted for review to the Advarra Institutional Review Board and received approval on 3 January, 2023 (ID: Pro00067958). Consent to participate: Participants provided written informed consent before any study activities were initiated. Participants were informed that this study was being completed for research purposes. Participants were informed that the anonymized coded data and transcripts would be analyzed and shared. Participants were informed that every effort would be made to report their information in a way that protects their privacy by avoiding any mention of their name or other information that could identify them. Consent for publication: Not applicable. Availability of data and material: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Code availability: Not applicable. Author contributions: All authors contributed to the study conception and/or design. Data collection and analysis were directed by EC, DB, CC, and HK. The first draft of the manuscript was written by EC, DB, and CC. All authors commented on and revised the draft manuscript. All authors read and approved the final manuscript.
Figures

References
-
- World Health Organization. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 1 Aug 2024.
-
- American Medical Association. Report of the Council on science and public health: is obesity a disease? https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/pub.... Accessed 1 Aug 2024.
-
- Mertens IL, Van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obes Res. 2000;8(3):270–8. 10.1038/oby.2000.32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

