Myasthenia gravis in 2025: five new things and four hopes for the future
- PMID: 39987373
- PMCID: PMC11846739
- DOI: 10.1007/s00415-025-12922-7
Myasthenia gravis in 2025: five new things and four hopes for the future
Abstract
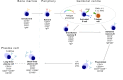
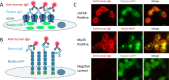
The last 10 years has brought transformative developments in the effective treatment of myasthenia gravis (MG). Beginning with the randomized trial of thymectomy in myasthenia gravis that demonstrated efficacy of thymectomy in nonthymomatous MG, several new treatment approaches have completed successful clinical trials and regulatory launch. These modalities, including B cell depletion, complement inhibition, and blockade of the neonatal Fc receptor, are now in use, offering prospects of sustained remission and neuromuscular protection in what is a long-term disease. In this review, we update our clinico-immunological review of 2016 with these important advances, examine their role in treatment algorithms, and focus attention on key issues of biomarkers for prognostication and the growing cohort of older patients, both those with long-term disease, and late-onset MG ('LOMG'). We close by expressing our four hopes for the next 5-10 years: improvements in laboratory medicine to facilitate rapid diagnosis, effective strategies for neuromuscular protection, more research into and better understanding of pathophysiology and treatment response in older individuals, and the potentially transformative role of therapies aimed at delivering a durable response such as chimeric antigen receptor (CAR) T cells. Our postscript summarizes some emerging themes in the field of serological and online biomarkers, which may develop greater stature in the next epoch.
Keywords: Clinical guidelines; Myasthenia gravis; Neuromuscular junction; Older adults; Rituximab; Thymectomy.
© 2025. Crown.
Conflict of interest statement
Declarations. Conflict of interest: IMM and MA have no nothing to declare.
Figures




References
-
- Barohn RJ, McIntire D, Herbelin L, et al (1998) Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci 841:769–772. 10.1111/j.1749-6632.1998.tb11015.x - PubMed
-
- Bedlack RS, Simel DL, Bosworth HSDB (2005) Myasthenia gravis score : assessment of responsiveness and. Neurology 64:1968–1970 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

