Bevacizumab beyond progression: Impact of subsequent bevacizumab re-treatment in patients with ovarian, fallopian tube, and peritoneal cancer after progression
- PMID: 39987772
- PMCID: PMC12115928
- DOI: 10.1016/j.ygyno.2025.02.015
Bevacizumab beyond progression: Impact of subsequent bevacizumab re-treatment in patients with ovarian, fallopian tube, and peritoneal cancer after progression
Abstract
Background: This study evaluated whether patients with epithelial ovarian, fallopian tube, and primary peritoneal carcinoma (OC) who are immediately re-treated with bevacizumab derive benefit after disease progression on a bevacizumab-containing regimen.
Methods: This multi-institutional, retrospective study compared patients with high grade non-mucinous epithelial OC who received bevacizumab followed directly by another bevacizumab-containing treatment regimen to patients who received bevacizumab followed by a regimen that did not contain bevacizumab (or received no further treatment). Progression-free survival (PFS) and overall survival (OS) were estimated using Kaplan Meier product-limit estimator and modeled via Cox proportional hazards regression.
Results: Among 226 patients with OC who received bevacizumab as part of a treatment regimen,103 received sequential treatment with bevacizumab and 123 received a bevacizumab-containing regimen followed by a non-bevacizumab-containing regimen at the time of progression. Median follow-up for all subjects was 17.3 months (range, 1.2-138.2 months). Median PFS was 17.2 months (95 % CI, 14.3-21.2) for patients who received sequential bevacizumab re-treatment and 5.1 months (95 % CI, 4.3-6.3) for patients who received bevacizumab without bevacizumab-containing re-treatment (p < 0.001). Median OS was 29.9 months (95 % CI, 26.1-35.4) for patients who received sequential bevacizumab re-treatment (p < 0.001) and 12.4 months (95 % CI, 9.2-16.7) for patients who did not receive bevacizumab-containing re-treatment.
Conclusion: Patients with OC treated with bevacizumab-containing regimens sequentially at the time of progression have prolonged survival compared to patients who received no re-treatment with bevacizumab.
Keywords: Angiogenesis inhibitors; Bevacizumab; Fallopian tube cancer; Ovarian cancer.
Copyright © 2025 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest RP has received consulting fees from Myriad Genetics and Natera. RP is employed by Labcorp. SNW has received research support to her institution from AstraZeneca, AvengeBio, Bayer, Bio-Path, Clovis Oncology/Pharm&, GSK, Jazz Pharmaceuticals, Mereo, Novartis, Nuvectis, Roche/Genentech, Zentalis and consulting fees from AstraZeneca, Caris, Clovis Oncology/Pharma&, Eisai, EQRX, Gilead, GSK, Immunocore, ImmunoGen, Lilly, Loxo, Merck, Mereo, Mersana, NGM Bio, Roche/Genentech, Seagen, Verastem, Vincerx, Zentalis, and ZielBio. AKS: shareholder of BioPath and a consultant for Merck, AstraZeneca, Onxeo, ImmunoGen, Ivlon, GSK, and Kiyatec. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. The Institutional Review Boards and relevant regulatory bodies at The University of Texas MD Anderson Cancer Center and Duke University Medical Center approved this study. Editorial support was provided by Bryan Tutt, Scientific Editor, Research Medical Library MD Anderson Cancer Center.
Figures

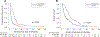

References
-
- SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute; 2023. Apr 19. [updated: 2023 Nov 16; cited 2024 Apr 16]. Available from: https://seer.cancer.gov/statistics-network/explorer/. Data source(s): U.S. Mortality Data (1969–2020), National Center for Health Statistics, CDC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

