Foamy monocytes and atherogenesis in mice with combined hyperlipidemia and effects of antisense knockdown of apoCIII
- PMID: 39988193
- PMCID: PMC11981816
- DOI: 10.1016/j.jlr.2025.100763
Foamy monocytes and atherogenesis in mice with combined hyperlipidemia and effects of antisense knockdown of apoCIII
Abstract
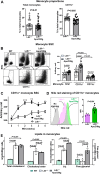
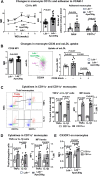
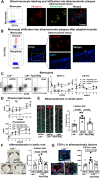
Hypertriglyceridemia (HTG), particularly in combined hyperlipidemia, increases risk for atherosclerotic cardiovascular disease, but the underlying mechanisms remain incompletely understood. We sought to determine contributions of circulating monocytes to atherosclerosis associated with HTG in combined hyperlipidemia, created by transgenic expression of human apoCIII in Ldlr-/- mice (Ldlr-/-ApoCIIItg) fed Western high-fat diet (WD). Tissue culture with THP-1 and primary human monocytes was used to examine effects of triglyceride (TG)-rich lipoproteins on monocytes. Ldlr-/-ApoCIIItg mice were also treated with apoCIII antisense oligonucleotide (ASO) and examined for foamy monocytes and atherosclerosis. Compared to Ldlr-/- mice, Ldlr-/-ApoCIIItg mice fed WD had early and persistent increases in lipid accumulation within monocytes and enhanced atherosclerosis. Ldlr-/-ApoCIIItg mice versus Ldlr-/- mice had higher levels of CD11c, CD36, and cytokines in foamy monocytes, with increases in foamy monocyte adhesion to vascular cell adhesion molecule-1 and oxidized LDL uptake. Monocytes took up TG-rich lipoprotein in vivo and in vitro and changed phenotypes. Foamy monocytes infiltrated into atherosclerotic lesions, and specific and sustained depletion of CD11c+ (foamy) monocytes profoundly reduced atherosclerosis in Ldlr-/-ApoCIIItg mice on WD. Treatment with apoCIII ASO lowered plasma TG and cholesterol levels, improved foamy monocyte phenotypes, and reduced atherosclerosis in Ldlr-/-ApoCIIItg mice. In conclusion, HTG in combined hyperlipidemia accelerates atherosclerosis, in part, by increasing foamy monocyte formation and infiltration into atherosclerotic plaques. Treatment with apoCIII ASO is a potential new therapy for improving monocyte phenotypes and reducing atherosclerosis in combined hyperlipidemia.
Keywords: atherosclerosis; cholesterol; foam cells; inflammation; lipoproteins; monocytes; triglyceride.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest Dr Ballantyne has received grant/research support through the institution from Ionis Pharmaceuticals and Arrowhead and consultant fees from Ionis Pharmaceuticals and Arrowhead. Dr Mullick and Dr Crooke are employees of Ionis Pharmaceuticals and provided the GalNac-conjugated ASOs against human or mouse apoCIII and CO used in this study. Ionis Pharmaceuticals did not provide financial support for this study. All other authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Ballantyne C.M., Olsson A.G., Cook T.J., Mercuri M.F., Pedersen T.R., Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104:3046–3051. - PubMed
-
- Miller M., Stone N.J., Ballantyne C.M., Bittner V., Criqui M.H., Ginsberg H.N., et al. Triglycerides and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2011;123:2292–2333. - PubMed
-
- Jorgensen A.B., Frikke-Schmidt R., Nordestgaard B.G., Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 2014;371:32–41. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

