Continued nintedanib in patients with systemic sclerosis-associated interstitial lung disease: 3-year data from SENSCIS-ON
- PMID: 39988350
- PMCID: PMC11848673
- DOI: 10.1136/rmdopen-2024-005086
Continued nintedanib in patients with systemic sclerosis-associated interstitial lung disease: 3-year data from SENSCIS-ON
Abstract
Objective: We assessed adverse events and changes in forced vital capacity (FVC) in patients treated with open-label nintedanib over 148 weeks of SENSCIS-ON, the extension of the SENSCIS trial.
Methods: Adverse events and changes in FVC over 148 weeks of SENSCIS-ON were assessed in patients who received nintedanib in SENSCIS and continued nintedanib in SENSCIS-ON ('continued nintedanib' group) and in patients who received placebo in SENSCIS or received nintedanib for ≤28 days in a drug-drug interaction study and then received nintedanib in SENSCIS-ON ('initiated nintedanib' group).
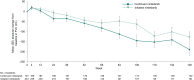
Results: The continued nintedanib group comprised 197 patients, and the initiated nintedanib group comprised 247 patients (231 from SENSCIS). Diarrhoea was the most frequent adverse event, reported in 152 (77.2%) and 183 (74.1%) patients in the continued nintedanib and initiated nintedanib groups, respectively. Among patients in the continued and initiated nintedanib groups, respectively, 53 (26.9%) and 148 (59.9%) had ≥1 dose reduction, 72 (36.5%) and 131 (53.0%) had ≥1 treatment interruption and 29 (14.7%) and 72 (29.1%) had adverse events that led to treatment discontinuation. Mean (SE) changes in FVC (mL) at week 148 were -189.1 (29.5) in the continued nintedanib group and -126.4 (26.4) in the initiated nintedanib group.
Conclusion: The safety profile of nintedanib over 148 weeks of SENSCIS-ON was consistent with that reported in SENSCIS. Changes in FVC during SENSCIS and SENSCIS-ON supported a continued effect of nintedanib on slowing the decline in lung function, but showed continued progression of SSc-ILD.
Keywords: Connective Tissue Diseases; Pulmonary Fibrosis; Scleroderma, Systemic.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: YA has acted as an advisor or review panel member for AstraZeneca, Boehringer Ingelheim, Chemomab, Curzion, Medsenic, Menarini, Prometheus, Sanofi; as a consultant for Boehringer Ingelheim and Sanofi; and as a speaker for AbbVie, Boehringer Ingelheim, Janssen. MCV has received research support from Boehringer Ingelheim, Ferrer, Galapagos, Janssen; acts as a speaker for Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novartis; and is a board member for EUSTAR and Systemic Sclerosis ERN ReCONNET. OD has/had consultancy relationship with and/or has received research funding from and/or has served as a speaker for the following companies in the area of potential treatments for SSc and its complications in the last three years: 4P-Pharma, Abbvie, Acceleron, Alcimed, Altavant, Amgen, AnaMar, Argenx, Arxx, AstraZeneca, Blade, Bayer, Boehringer Ingelheim, Cantargia, Catalyze Capital, Corbus, CSL Behring, Galderma, Galapagos, Glenmark, Gossamer, Horizon, Janssen, Kymera, Lupin, Medscape, Merck Sharp & Dohme, Miltenyi Biotec, Mitsubishi Tanabe, Nkarta, Novartis, Orion, Prometheus, Redx, Roivant, EMD Serono, Topadur, UCB; he has a patent-issued 'mir-29 for the treatment of systemic sclerosis' (US8247389, EP2331143) and is a co-founder of CITUS AG. AA has acted as a consultant for Boehringer Ingelheim, Kyorin Pharma, Taiho, Toray, and has acted as a speaker and received research support from Boehringer Ingelheim. MDM has received research support from Boehringer Ingelheim, Corbus, Eicos, Horizon Pharma, Mitsubishi Tanabe, Prometheus and royalties from the British Medical Journal, Oxford University Press and Springer International Publishing; has acted as a speaker for Medtelligence; and is as an advisor or review panel member for Boehringer Ingelheim, EICOS, Mitsubishi Tanabe. DK has acted as a consultant for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Genentech, Horizon Therapeutics, Janssen, Prometheus, UCB; as a speaker for Jannsen and as a member of a data safety monitoring board for AbbVie; has received research support from Bristol Myers Squibb, Horizon Therapeutics, Pfizer and royalties for the University of California Los Angeles Scleroderma Clinical Trials Consortium (SCTC) Gastrointestinal Tract instrument 2.0. KBH has acted as a consultant and speaker for and received research support from Boehringer Ingelheim and acted as an advisor or review panel member for the Scleroderma Foundation. AJ is a consultant to Elderbrook Solutions, which was contracted by Boehringer Ingelheim to conduct the analyses presented in this manuscript, and is also a consultant for AbbVie. VK and MA are employees of Boehringer Ingelheim.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous