Autologous iPSC- and MSC-derived chondrocyte implants for cartilage repair in a miniature pig model
- PMID: 39988676
- PMCID: PMC11849328
- DOI: 10.1186/s13287-025-04215-7
Autologous iPSC- and MSC-derived chondrocyte implants for cartilage repair in a miniature pig model
Abstract
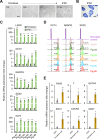
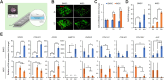
Background: Induced pluripotent stem cell (iPSC)-derived mesenchymal stem cells (iMSCs) have greater potential for generating chondrocytes without hypertrophic and fibrotic phenotypes compared to bone marrow-derived mesenchymal stem/stromal cells (BMSCs). However, there is a lack of research demonstrating the use of autologous iMSCs for repairing articular chondral lesions in large animal models. In this study, we aimed to evaluate the effectiveness of autologous miniature pig (minipig) iMSC-chondrocyte (iMSC-Ch)-laden implants in comparison to autologous BMSC-chondrocyte (BMSC-Ch)-laden implants for cartilage repair in porcine femoral condyles.
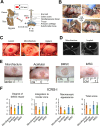
Methods: iMSCs and BMSCs were seeded into fibrin glue/nanofiber constructs and cultured with chondrogenic induction media for 7 days before implantation. To assess the regenerative capacity of the cells, 19 skeletally mature Yucatan minipigs were randomly divided into microfracture control, acellular scaffold, iMSC, and BMSC subgroups. A cylindrical defect measuring 7 mm in diameter and 0.6 mm in depth was created on the articular cartilage surface without violating the subchondral bone. The defects were then left untreated or treated with acellular or cellular implants.
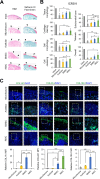
Results: Both cellular implant-treated groups exhibited enhanced joint repair compared to the microfracture and acellular control groups. Immunofluorescence analysis yielded significant findings, showing that cartilage treated with iMSC-Ch implants exhibited higher expression of COL2A1 and minimal to no expression of COL1A1 and COL10A1, in contrast to the BMSC-Ch-treated group. This indicates that the iMSC-Ch implants generated more hyaline cartilage-like tissue compared to the BMSC-Ch implants.
Conclusions: Our findings contribute to filling the knowledge gap regarding the use of autologous iPSC derivatives for cartilage repair in a translational animal model. Moreover, these results highlight their potential as a safe and effective therapeutic strategy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: The animals used and the experimental procedures conducted in this study adhered to an animal protocol approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. The protocol titled “Cartilage Implants in a Pig Model” with ID# V005016-R02 received approval on June 8th, 2020. Consent for publication: No consent is required for publication. Competing interests: The authors declare no competing interests.
Figures


References
-
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–63. - PubMed
-
- Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36(2):235–46. - PubMed
-
- Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105–12. - PubMed
-
- Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432–63. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

