Guidelines for the use of lung ultrasound to optimise the management of neonatal respiratory distress: international expert consensus
- PMID: 39988689
- PMCID: PMC11849336
- DOI: 10.1186/s12916-025-03879-5
Guidelines for the use of lung ultrasound to optimise the management of neonatal respiratory distress: international expert consensus
Abstract
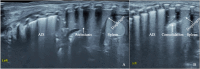
Background: Respiratory distress is the main reason for the admission of infants to the neonatal intensive care unit (NICU). Rapid identification of the causes of respiratory distress and selection of appropriate and effective treatment strategies are important to optimise favourable short- and long-term patient outcomes. Lung ultrasound (LUS) technology has become increasingly important in this field. According to the scientific literature, LUS has high sensitivity (92-99%) and specificity (95-97%) in diagnosing neonatal respiratory distress syndrome. This diagnostic power helps guide timely interventions, such as surfactant therapy and mechanical ventilation.
Methods: Our objective was to outline consensus guidelines among an international panel of experts on the use of LUS to support the decision-making process in managing respiratory distress in the NICU. We used a three-round Delphi process. In each Delphi round, 28 panellists rated their level of agreement with each statement using a four-point Likert scale.
Results: In round 1, the panellists reviewed 30 initially proposed statements. In rounds 2 and 3, the statements were redeveloped based on the reviewers' comments, leading to the final approval of 18 statements. Among the 18 consensus statements, grade A was assigned a value of 10, grade B was assigned a value of 7, and grade C was assigned a value of 1.
Conclusions: A panel of experts agreed on 18 statements regarding managing infants with respiratory distress. Using LUS may help design future interventional studies and improve the benchmarking of respiratory care outcomes.
Keywords: Diagnostic Imaging; Dyspnoea; Lung ultrasound; Mechanical ventilation; Neonatal intensive care unit; Neonate; Pulmonary surfactant; Respiratory distress.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This work is a methodological study that does not involve human or animal subjects and does not require ethical approval. Informed consent from guardians or study subjects was not needed. Consent for publication: Informed consent was obtained from all individual participants included in the study. Competing interests: The authors declare no competing interests.
Figures









References
-
- Jain D, Bancalari E. New developments in respiratory support for preterm infants. Am J Perinatol. 2019;36(S02):S13–7. - PubMed
-
- Liu G, Wu H, Li Z. Current views of complications associated with neonatal ventilation. Minerva Pediatr. 2020;72:60–4. - PubMed
-
- Kohbodi GA, Rajasurya V, Noor A. Ventilator-associated pneumonia. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. - PubMed
-
- Cernada M, Brugada M, Golombek S, Vento M. Ventilator-associated pneumonia in neonatal patients: an update. Neonatology. 2014;105:98–107. - PubMed
-
- Solberg MT, Solevåg AL, Clarke S. Optimal conventional mechanical ventilation in full-term newborns: a systematic review. Adv Neonatal Care. 2018;18:451–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

