Population Pharmacokinetics of Cefepime in Critically Ill Children and Young Adults: Model Development and External Validation for Monte Carlo Simulations and Model-Informed Precision Dosing
- PMID: 39988706
- PMCID: PMC12041147
- DOI: 10.1007/s40262-025-01485-5
Population Pharmacokinetics of Cefepime in Critically Ill Children and Young Adults: Model Development and External Validation for Monte Carlo Simulations and Model-Informed Precision Dosing
Abstract
Background and objective: This study aimed to develop a population pharmacokinetic model for cefepime in critically ill pediatric and young adult patients to inform dosing recommendations and to evaluate the model's predictive performance for model-informed precision dosing.
Methods: Patients in the pediatric intensive care unit receiving cefepime were prospectively enrolled for clinical data collection and opportunistic plasma sampling for cefepime concentrations. Nonlinear mixed effects modeling was conducted using NONMEM. Allometric body weight scaling was included as a covariate with fixed exponents. Monte Carlo simulations determined optimal initial dosing regimens against susceptible pathogens. The model's predictions were evaluated with an external dataset.
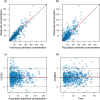
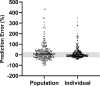
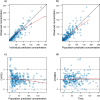
Results: Data from 510 samples across 100 patients were best fit with a two-compartment model with first-order elimination. Estimated glomerular filtration rate and cumulative percentage of fluid balance were identified as significant covariates on clearance and central volume of distribution, respectively. Internal validation showed no model misspecification. External validation confirmed that bias and precision for both population and individual predictions were within commonly accepted ranges. Monte Carlo simulations suggested that the usual dose of 50 mg/kg may require a 3-h infusion or a 6-h dosing interval to keep concentrations above the Pseudomonas aeruginosa minimum inhibitory concentration (≤ 8 mg/L) throughout the dosing interval for patients with normal or augmented renal clearance.
Conclusion: A cefepime population pharmacokinetic model for critically ill pediatric patients was successfully developed, accounting for patient renal function, fluid status, and body size, using real-world data. The model was internally and externally validated for use in optimal dosing simulations and model-informed precision dosing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent to participate: The study was approved by the Institutional Review Board with a waiver of consent. Consent for publication: Not applicable. Code availability: The model codes that support the findings of this study are accessible upon reasonable request by contacting the corresponding author. Data availability: The data and material are accessible upon reasonable request by contacting the corresponding author. Ethics approval: This research included two studies approved by the CCHMC institutional review board with waiver of consent: “Pharmacokinetics of β-lactams in Critically Ill Pediatric Patients during Different Stages of Sepsis” (IRB #2018-3245), and “Taking Focus 2” (IRB #2018-0724). Authors’ contributions: R Morales Junior, HR Hambrick, and S Tang Girdwood conceptualized the study. HR Hambrick, KE Pavia, KM Paice, KA Krallman, L Johnson, M Collins, A Gibson, and C Curry collected the data. P Tang and E Schuler conducted the laboratory analyses. R Morales Junior, T Mizuno, and S Tang Girdwood analyzed the pharmacokinetic data. R Morales Junior and HR Hambrick wrote the original draft of the manuscript, and all authors contributed to reviewing and editing the manuscript. J Kaplan, S Goldstein, and S Tang Girdwood supervised the project. Funding: This work was generously supported by the National Institutes of Health, including funding from the National Institute of General Medical Sciences under an R35 award (R35GM14670), the Eunice Kennedy Shriver National Institute of Child Health and Human Development T32 Training Program in Pediatric Clinical and Developmental Pharmacology (T32HD069054), and the National Institute of Diabetes and Digestive and Kidney Diseases (T32DK007695). Conflict of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Rybak M. The pharmacokinetic profile of a new generation of parenteral cephalosporin. Am J Med. 1996;100:39S-44S. - PubMed
-
- Fratoni AJ, Nicolau DP, Kuti JL. A guide to therapeutic drug monitoring of β-lactam antibiotics. Pharmacotherapy. 2021;41:220–33. - PubMed
-
- Cies JJ, Moore WS, Enache A, Chopra A. β-Lactam therapeutic drug management in the PICU. Crit Care Med. 2018;46:272–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

