Dupilumab Efficacy and Safety in Patients With Persistent Asthma: Asia-Pacific Region
- PMID: 39988927
- PMCID: PMC12325141
- DOI: 10.1111/cea.70005
Dupilumab Efficacy and Safety in Patients With Persistent Asthma: Asia-Pacific Region
Abstract
Background: Asthma prevalence is increasing in the Asia-Pacific region. China and India account for > 35% of the world's population and are often underrepresented in clinical studies. This phase 3 study (NCT03782532) evaluated efficacy and safety of dupilumab, a monoclonal antibody blocking interleukin-4/13 signalling, in patients with persistent asthma from China and India.
Methods: Patients (≥ 12 years) were randomised 1:1 to dupilumab 200 mg or matched placebo every 2 weeks for 24 weeks (primary analysis population: blood eosinophils ≥ 150 cells/μL or fractional exhaled nitric oxide ≥ 25 parts per billion without maintenance oral corticosteroid [OCS]; OCS maintenance population: 300 mg OCS).
Primary endpoint: change from baseline to week 12 in forced expiratory volume in 1 s (FEV1). Secondary endpoints: change from baseline to week 24 in 5-item Asthma Control Questionnaire (ACQ-5/7) scores, annualised severe exacerbation rate, and safety.
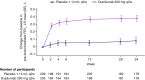
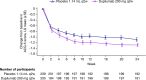
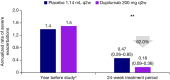
Results: In the primary analysis population (n = 414), change in FEV1 by week 12 was significantly greater for dupilumab versus placebo (least squares mean difference: 0.31 L [95% CI: 0.23-0.39]; p < 0.0001). At week 24, greater reductions in ACQ-5 score were seen for dupilumab versus placebo (least squares mean difference: -0.20 [95% CI: -0.35 to -0.05]; p = 0.0097). Dupilumab reduced severe exacerbation risk by 62% versus placebo during the treatment period (relative risk: 0.38 [95% CI: 0.21-0.70]; nominal p = 0.002). Safety was similar between treatment arms; injection-site reactions were more common with dupilumab treatment (5.0%) than with placebo (1.2%). The OCS maintenance population showed similar outcomes.
Conclusion: Dupilumab significantly improved lung function and asthma control, numerically reduced asthma exacerbations, and was well tolerated in patients from China and India with persistent asthma and evidence of either type 2 inflammation or OCS maintenance.
Trial registration: ClinicalTrials.gov identifier: NCT03782532.
Keywords: Asia–Pacific; asthma exacerbations; lung function; monoclonal antibody; type 2 inflammation.
© 2025 The Author(s). Clinical & Experimental Allergy published by John Wiley & Sons Ltd.
Conflict of interest statement
Q.Z., N.Z., S.D., X.F., H.F., J.L. and S.Z. declare no conflicts of interest. E.L., Y.W., V.L., C.‐C.H. and L.B.R. are Sanofi employees, and may hold stock and/or stock options in the company. J.M. is an employee and shareholder of Regeneron Pharmaceuticals Inc. R.M.A. is a former Sanofi employee, and may hold stock and/or stock options in the company.
Figures
References
-
- Global Initiative for Asthma , “GINA Report, Global Strategy for Asthma Management and Prevention,” (2023), https://ginasthma.org/wp‐content/uploads/2023/07/GINA‐2023‐Full‐report‐2....
-
- Global Asthma Network , “The Global Asthma Report 2022,” (2022), http://globalasthmareport.org/resources/Global_Asthma_Report_2022.pdf.
-
- Huang K., Yang T., Xu J., et al., “Prevalence, Risk Factors, and Management of Asthma in China: A National Cross‐Sectional Study,” Lancet 394 (2019): 407–418. - PubMed

