Quantifying the relationship between cell proliferation and morphology during development of the face
- PMID: 39989423
- PMCID: PMC12045601
- DOI: 10.1242/dev.204511
Quantifying the relationship between cell proliferation and morphology during development of the face
Abstract
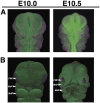
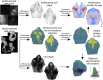
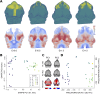
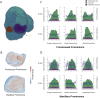
Morphogenesis requires highly coordinated, complex interactions between cellular processes: proliferation, migration and apoptosis, along with physical tissue interactions. How these cellular and tissue dynamics drive morphogenesis remains elusive. Three dimensional (3D) microscopic imaging holds great promise, and generates elegant images, but generating even moderate throughput for quantified images is challenging for many reasons. As a result, the association between morphogenesis and cellular processes in 3D developing tissues has not been fully explored. To address this gap, we have developed an imaging and image analysis pipeline to enable 3D quantification of cellular dynamics along with 3D morphology for the same individual embryo. Specifically, we focus on how 3D distribution of proliferation relates to morphogenesis during mouse facial development. Our method involves imaging with light-sheet microscopy, automated segmentation of cells and tissues using machine learning-based tools, and quantification of external morphology by geometric morphometrics. Applying this framework, we show that changes in proliferation are tightly correlated with changes in morphology over the course of facial morphogenesis. These analyses illustrate the potential of this pipeline to investigate mechanistic relationships between cellular dynamics and morphogenesis during embryonic development.
Keywords: Convolutional neural networks; Developmental biology; Image segmentation; Light-sheet imaging; Morphometrics; Mouse embryo.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Update of
-
Quantifying the relationship between cell proliferation and morphology during development of the face.bioRxiv [Preprint]. 2023 May 12:2023.05.12.540515. doi: 10.1101/2023.05.12.540515. bioRxiv. 2023. Update in: Development. 2025 Apr 01;152(7):dev204511. doi: 10.1242/dev.204511. PMID: 37214859 Free PMC article. Updated. Preprint.
Similar articles
-
Quantifying the relationship between cell proliferation and morphology during development of the face.bioRxiv [Preprint]. 2023 May 12:2023.05.12.540515. doi: 10.1101/2023.05.12.540515. bioRxiv. 2023. Update in: Development. 2025 Apr 01;152(7):dev204511. doi: 10.1242/dev.204511. PMID: 37214859 Free PMC article. Updated. Preprint.
-
A dynamic and expandable digital 3D-atlas maker for monitoring the temporal changes in tissue growth during hindbrain morphogenesis.Elife. 2022 Sep 28;11:e78300. doi: 10.7554/eLife.78300. Elife. 2022. PMID: 36169400 Free PMC article.
-
Morphometrics, 3D Imaging, and Craniofacial Development.Curr Top Dev Biol. 2015;115:561-97. doi: 10.1016/bs.ctdb.2015.09.003. Epub 2015 Oct 27. Curr Top Dev Biol. 2015. PMID: 26589938 Free PMC article. Review.
-
An ex vivo system to study cellular dynamics underlying mouse peri-implantation development.Dev Cell. 2022 Feb 7;57(3):373-386.e9. doi: 10.1016/j.devcel.2021.12.023. Epub 2022 Jan 20. Dev Cell. 2022. PMID: 35063082 Free PMC article.
-
Quantitative analysis of cell shape and the cytoskeleton in developmental biology.Wiley Interdiscip Rev Dev Biol. 2018 Nov;7(6):e333. doi: 10.1002/wdev.333. Epub 2018 Aug 31. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 30168893 Free PMC article. Review.
References
-
- Alladin, A., Chaible, L., Garcia del Valle, L., Sabine, R., Loeschinger, M., Wachsmuth, M., Hériché, J.-K., Tischer, C. and and Jechlinger, M. (2020). Tracking cells in epithelial acini by light sheet microscopy reveals proximity effects in breast cancer initiation. eLife 9, e54066. 10.7554/eLife.54066 - DOI - PMC - PubMed
-
- Baken, E., Collyer, M., Kaliontzopoulou, A. and Adams, D. (2021). geomorph v4.0 and gmShiny: enhanced analytics and a new graphical interface for a comprehensive morphometric experience. Methods Ecol. Evol. 12, 2355-2363. 10.1111/2041-210X.13723 - DOI
MeSH terms
Grants and funding
- Compute Canada
- CAPMC/ CIHR/Canada
- Institute of Musculoskeletal Health and Arthritis
- Alberta Children's Hospital Foundation
- RGPIN-2024-06098/Canada Research Chairs
- R01 DE019638/DE/NIDCR NIH HHS/United States
- R01-DE019638/DE/NIDCR NIH HHS/United States
- 36262/Canada Foundation for Innovation
- University of Calgary
- 238992/Natural Sciences and Engineering Research Council of Canada
- University of Pittsburgh
- Cumming School of Medicine, University of Calgary
- RGPIN-2024-06098/Natural Sciences and Engineering Research Council of Canada
- Western Canada Research Grid
- Alberta Innovates
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

