Virologic effects of broadly neutralizing antibodies VRC01LS and VRC07-523LS on chronic HIV-1 infection
- PMID: 39989458
- PMCID: PMC11949028
- DOI: 10.1172/jci.insight.181496
Virologic effects of broadly neutralizing antibodies VRC01LS and VRC07-523LS on chronic HIV-1 infection
Abstract
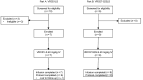
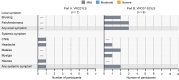
BACKGROUNDHIV-1-specific broadly neutralizing monoclonal antibodies (bNAbs) have emerged as promising interventions with the potential to effectively treat and prevent HIV-1 infections. We conducted a phase I clinical trial evaluating the potent CD4-binding site-specific (CD4bs-specific) bNAbs VRC01LS and VRC07-523LS in people with HIV-1 (PWH) not receiving antiretroviral therapy (ART).METHODSParticipants received a single intravenous 40 mg/kg dose of either VRC01LS (n = 7) or VRC07-523LS (n = 9) and did not initiate ART for a minimum of 14 days. The primary study objective was to evaluate safety and tolerability; the secondary study objectives were to evaluate pharmacokinetics (PK) and the impact of administered bNAbs on viral loads (VL) and CD4+ T cell counts in the absence of ART.RESULTSThis trial enrolled 16 PWH aged 20 to 57 years. Both bNAbs were safe and well tolerated. Mild local reactogenicity was only reported in participants who received VRC07-523LS, while both bNAbs were associated with mild systemic symptoms. Maximum serum concentrations (Cmax) following VRC01LS or VRC07-523LS were 1,566 ± 316 and 1,295 ± 376 μg/mL, respectively. VRC07-523LS administration significantly decreased VL in 8 out of 9 participants, with an average decline of 1.7 ± 0.8 log10 copies/mL within 14 days after administration. In contrast, VRC01LS administration resulted in a smaller average decline (0.8 ± 0.8 log10 copies/mL), and 3 out of 7 participants showedno change in VL. Postinfusion maximum decline in VL correlated with post hoc baseline in vitro viral susceptibility results for both bNAbs.CONCLUSIONThe results of this trial support inclusion of potent CD4bs-specific bNAbs, such as VRC07-523LS, into next-generation treatment regimens for HIV-1.TRIAL REGISTRATIONClinicalTrials.gov NCT02840474.FUNDINGNational Institute of Allergy and Infectious Diseases (NIAID)/NIH (grants UM1 AI068634, UM1 AI068636, UM1 AI106701, UM1AI069424, UM1AI069501, UM1AI69415, UM1AI069534, UM1AI69494); the Intramural Research Program of the NIAID/NIH; National Center for Advancing Translational Sciences/NIH (grants UM1TR004548, UL1TR001881, and UL1TR001878); and the National Cancer Institute/NIH (contract 75N91019D00024).
Keywords: AIDS/HIV; Clinical trials; Drug therapy.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- UM1 AI069415/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- 75N91019D00024/CA/NCI NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 TR004548/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

