Purification and characterization of detergent stable alkaline lipase from Bacillus safensis TKW3 isolated from Tso Kar brackish water lake
- PMID: 39989736
- PMCID: PMC11846503
- DOI: 10.7717/peerj.18921
Purification and characterization of detergent stable alkaline lipase from Bacillus safensis TKW3 isolated from Tso Kar brackish water lake
Abstract
Extensive and escalating research has been directed towards halozymes derived from halophiles thriving in extreme hypersaline environments, owing to their myriad industrial applications. These extremophiles have evolved various physiological and metabolic adaptations to endure such extremes, enhancing their industrial potential. Being a potential source of lipases, halophiles of extreme niches have emerged as a emerging research area. This interest has been fueled by the recognition that extreme environments serve as rich reservoirs of diverse cold-active alkaliphilic enzymes.
Methods: Bacillus safensis TKW3, isolated from brackish Lake Tso Kar of the Ladakh region, India, produced cold-adapted haloalkaliphilic lipase halozyme. The current study focused on the purification and biochemical characterisation of lipase derived from halophilic bacteria.
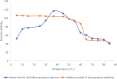
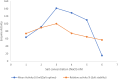
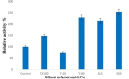
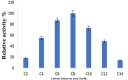
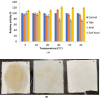
Results: The lipase enzyme, purified to homogeneity, exhibited a molecular mass of 28 kDa as confirmed by SDS-PAGE analysis. The purification process yielded a purification fold of 12.01 and a final recovery rate of 29.9%. It demonstrated optimal activity at 30 °C and pH 9. The enzyme was evaluated and demonstrated to exhibit stability over a broad temperature range spanning from 5 °C to 55 °C, as well as a wide pH range of 7.0 to 9.0. Due to its stability across a diverse spectrum of pH values, surfactants, metal ions, and inhibitors, the enzyme appeared to hold significant promise for application within the leather and detergent sectors. Upon undergoing detergent compatibility tests spanning diverse temperature ranges, the lipase showcased compatibility with various commercial detergents, thereby presenting itself as an attractive candidate for inclusion in detergent formulations within the industry.
Conclusions: The lipase from B. safensis TKW3 exhibits promising attributes, including alkali stability, halophilicity, and a wide spectrum of substrate specificity, rendering it an attractive option for incorporation into detergent formulations within the detergent industry. As far as we are aware, this is the first report on the purification and characterization of lipase enzyme from bacterial halophiles in a Tso Kar brackish lake.
Keywords: Alkaline; B. safensis; Cold-adapted; Detergent compatible; Halophilic; Lipase.
© 2025 Devi et al.
Conflict of interest statement
Srinivas Sistla is an Academic Editor for PeerJ.
Figures



References
-
- Barone R, De Santi C, Palma Esposito F, Tedesco P, Galati F, Visone M, De Pascale D. Marine metagenomics, a valuable tool for enzymes and bioactive compounds discovery. Frontiers in Marine Science. 2014;1:38. doi: 10.3389/fmars.2014.00038. - DOI
-
- Boutaiba S, Bhatnagar T, Hacene H, Mitchell D, Baratti J. Preliminary characterisation of a lipolytic activity from an extremely halophilic archaeon, Natronococcus sp. Journal of Molecular Catalysis B: Enzymatic. 2006;41(1–2):21–26. doi: 10.1016/j.molcatb.2006.03.010. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

