Environmentally Sustainable Approach of Corrosion Inhibition of Mild Steel in 1 N HCl and 1 N H2SO4 via Antihistamine Loratadine (LT) and Its Amine Derivatives: Computational and Experimental Analysis
- PMID: 39989764
- PMCID: PMC11840622
- DOI: 10.1021/acsomega.4c06238
Environmentally Sustainable Approach of Corrosion Inhibition of Mild Steel in 1 N HCl and 1 N H2SO4 via Antihistamine Loratadine (LT) and Its Amine Derivatives: Computational and Experimental Analysis
Abstract
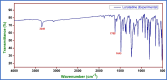
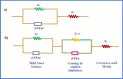
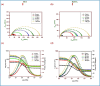
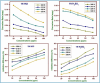
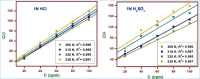
The efficacy of pharmaceuticals in mitigating corrosion on metallic substrates has led to the development of a new class of inhibitors that are economically efficient and offer significant environmental benefits. In the present study, for the anticorrosion action of loratadine (LT), 4-(8-chloro-5,6-dihydro-11H-benzo [5,6] cyclohepta[1,2-b] pyridin-11-ylidene)-1-piperidinecarboxylic acid ethyl ester and its amine derivatives (LT1, LT2, and LT3), a theoretical study using DFT was conducted to elucidate the molecular interactions and complex formation mechanisms between these inhibitors and mild steel. The ΔE values for the studied inhibitors-2.17 eV (LT), 3.904 eV (LT1), 3.906 eV (LT2), and 3.85 eV (LT3)-indicate that the LT inhibitor shows greater reactivity compared to the other LT amine derivatives, particularly in terms of electron donation to the metal substrate. After that, the corrosion inhibitory efficacy of parent molecule LT was examined on steel substrate in a medium 1 N hydrochloric and 1 N sulfuric acid and was found to have an efficiency of 98.52 and 80.58%, respectively, (308 K for 100 ppm concentration) deliberated through the electrochemical techniques (PDP and EIS) and gravimetric technique. The reduction in Cdl values from 684.06 to 43.15 μF/cm2 in 1 N HCl and from 693.41 to 83.91 μF/cm2 in 1 N H2SO4 indicates an adsorption process in which inhibitor molecules displace water adsorbed on the metallic substrate, creating a barrier that prevents the metallic substrate from corrosive damage. The surface adsorption aligned with the Langmuir adsorption isotherm with Gibbs-free energy -51 kJ/mol in 1 N HCl and -49.23 kJ/mol in 1 N H2SO4. The AFM analysis with an average roughness of 16.29 nm in HCl and 49.23 nm in H2SO4 validated LT to form a barrier on mild steel, opposing corrosion. The decelerative effect of LT inferred from theoretical and experimental data comply, making them credible corrosion inhibitors.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











Similar articles
-
Electrochemical and computational insights into the utilization of 2, 2- dithio bisbenzothiazole as a sustainable corrosion inhibitor for mild steel in low pH medium.Environ Res. 2024 Feb 1;242:117640. doi: 10.1016/j.envres.2023.117640. Epub 2023 Nov 24. Environ Res. 2024. PMID: 38007078
-
Synthesis of a new quaternary ammonium salt for efficient inhibition of mild steel corrosion in 15 % HCl: Experimental and theoretical studies.Heliyon. 2024 Sep 26;10(19):e38425. doi: 10.1016/j.heliyon.2024.e38425. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39416823 Free PMC article.
-
Investigating the Adsorption and Corrosion Protection Efficacy and Mechanism of Marjoram Extract on Mild Steel in HCl Medium.Molecules. 2025 Jan 11;30(2):272. doi: 10.3390/molecules30020272. Molecules. 2025. PMID: 39860142 Free PMC article.
-
Experimental and Quantum Chemical Investigations on the Anticorrosion Efficiency of a Nicotinehydrazide Derivative for Mild Steel in HCl.Molecules. 2022 Sep 23;27(19):6254. doi: 10.3390/molecules27196254. Molecules. 2022. PMID: 36234791 Free PMC article.
-
Sustainable technology for cultural heritage preservation: The role of green corrosion inhibitors.Sci Total Environ. 2025 May 1;975:179301. doi: 10.1016/j.scitotenv.2025.179301. Epub 2025 Apr 5. Sci Total Environ. 2025. PMID: 40187339 Review.
References
-
- Vashishth P.; Bairagi H.; Narang R.; Kumar H.; Mangla B. Efficacy of Biomass-Derived Nanocomposites as Promising Materials as Corrosion Inhibitors. Antiviral and Antimicrobial Coatings Based on Functionalized Nanomaterials: Design, Applications, and Devices 2023, 285–303. 10.1016/B978-0-323-91783-4.00007-3. - DOI
-
- Vashishth P.; Bairagi H.; Narang R.; Shukla S. K.; Olasunkanmi L. O.; Ebenso E. E.; Mangla B. Experimental Investigation of Sustainable Corrosion Inhibitor Albumin on Low-Carbon Steel in 1 N HCl and 1 N H2SO4. Results in Surfaces and Interfaces 2023, 13, 10015510.1016/j.rsurfi.2023.100155. - DOI
-
- Oubaaqa M.; Ouakki M.; Rbaa M.; Benhiba F.; Galai M.; Idouhli R.; Maatallah M.; Jarid A.; Warad I.; Lakhrissi B.; Zarrouk A.; Ebn Touhami M. Experimental and theoretical investigation of corrosion inhibition effect of two 8-hydroxyquinoline carbonitrile derivatives on mild steel in 1 M HCl solution. J. Phys. Chem. Solids. 2022, 169, 11086610.1016/j.jpcs.2022.110866. - DOI
-
- Akpan E. D.; Kumar Singh A.; Lgaz H.; Quadri T. W.; Kumar Shukla S.; Mangla B.; Dwivedi A.; Dagdag O.; Sheetal; Edem Inyang E.; Ebenso E. E. Coordination Compounds as Corrosion Inhibitors of Metals: A Review. Coord. Chem. Rev. 2024, 499, 21550310.1016/j.ccr.2023.215503. - DOI
-
- Obot I. B.; Obi-Egbedi N. O.; Umoren S. A. Antifungal Drugs as Corrosion Inhibitors for Aluminium in 0.1 M HCl. Corros. Sci. 2009, 51 (8), 1868–1875. 10.1016/j.corsci.2009.05.017. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
