Advancements in Micro/Nanorobots in Medicine: Design, Actuation, and Transformative Application
- PMID: 39989765
- PMCID: PMC11840590
- DOI: 10.1021/acsomega.4c09806
Advancements in Micro/Nanorobots in Medicine: Design, Actuation, and Transformative Application
Abstract
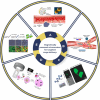
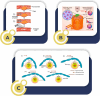
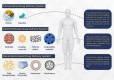
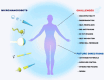
In light of the ongoing technological transformation, embracing advancements that foster shared benefits is essential. Nanorobots, a breakthrough within nanotechnology, have demonstrated significant potential in fields such as medicine, where diagnostic and therapeutic applications are the primary focus areas. This review provides a comprehensive overview of nanotechnology, robots, and their evolving role in medical applications, particularly highlighting the use of nanorobots. Various design strategies and operational principles, including sensors, actuators, and nanocontrollers, are discussed based on prior research. Key nanorobot medical applications include biomedical imaging, biosensing, minimally invasive surgery, and targeted drug delivery, each utilizing advanced actuation technologies to enhance precision. The paper further examines recent progress in micro/nanorobot actuation and addresses important considerations for the future, including biocompatibility, control, navigation, delivery, targeting, safety, and ethical implications. This review offers a holistic perspective on how nanorobots can reshape medical practices, paving the way for precision medicine and improved patient outcomes.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures












Similar articles
-
Biohybrid Micro/Nanorobots: Pioneering the Next Generation of Medical Technology.Adv Healthc Mater. 2024 Dec;13(31):e2402102. doi: 10.1002/adhm.202402102. Epub 2024 Oct 7. Adv Healthc Mater. 2024. PMID: 39373299 Free PMC article. Review.
-
Design and Control of the Magnetically Actuated Micro/Nanorobot Swarm toward Biomedical Applications.Adv Healthc Mater. 2024 Jun;13(15):e2400414. doi: 10.1002/adhm.202400414. Epub 2024 Mar 8. Adv Healthc Mater. 2024. PMID: 38412402 Review.
-
State of the Art in Actuation of Micro/Nanorobots for Biomedical Applications.Small Sci. 2024 Feb 2;4(3):2300211. doi: 10.1002/smsc.202300211. eCollection 2024 Mar. Small Sci. 2024. PMID: 40212697 Free PMC article.
-
Design, fabrication and application of magnetically actuated micro/nanorobots: a review.Nanotechnology. 2022 Jan 18;33(15). doi: 10.1088/1361-6528/ac43e6. Nanotechnology. 2022. PMID: 34915458 Review.
-
Application of micro/nanorobot in medicine.Front Bioeng Biotechnol. 2024 Jan 25;12:1347312. doi: 10.3389/fbioe.2024.1347312. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38333078 Free PMC article. Review.
Cited by
-
Future-Oriented Biomaterials Based on Natural Polymer Resources: Characteristics, Application Innovations, and Development Trends.Int J Mol Sci. 2025 Jun 9;26(12):5518. doi: 10.3390/ijms26125518. Int J Mol Sci. 2025. PMID: 40564982 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
