Hemodynamic disturbance and mTORC1 activation: Unveiling the biomechanical pathogenesis of thoracic aortic aneurysms in Marfan syndrome
- PMID: 39989903
- PMCID: PMC11847113
- DOI: 10.1016/j.jpha.2024.101120
Hemodynamic disturbance and mTORC1 activation: Unveiling the biomechanical pathogenesis of thoracic aortic aneurysms in Marfan syndrome
Abstract
Thoracic aortic aneurysm (TAA) significantly endangers the lives of individuals with Marfan syndrome (MFS), yet the intricacies of their biomechanical origins remain elusive. Our investigation delves into the pivotal role of hemodynamic disturbance in the pathogenesis of TAA, with a particular emphasis on the mechanistic contributions of the mammalian target of rapamycin (mTOR) signaling cascade. We uncovered that activation of the mTOR complex 1 (mTORC1) within smooth muscle cells, instigated by the oscillatory wall shear stress (OSS) that stems from disturbed flow (DF), is a catalyst for TAA progression. This revelation was corroborated through both an MFS mouse model (Fbn1 +/C1039G) and clinical MFS specimens. Crucially, our research demonstrates a direct linkage between the activation of the mTORC1 pathway and the intensity in OSS. Therapeutic administration of rapamycin suppresses mTORC1 activity, leading to the attenuation of aberrant SMC behavior, reduced inflammatory infiltration, and restoration of extracellular matrix integrity-collectively decelerating TAA advancement in our mouse model. These insights posit the mTORC1 axis as a strategic target for intervention, offering a novel approach to manage TAAs in MFS and potentially pave insights for current treatment paradigms.
Keywords: Biomechanicalpathogenesis; Marfan syndrome; Thoracic aortic aneurysm (TAA); Wall shear stress (WSS); mTORC1.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


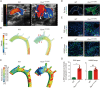
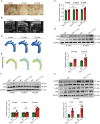



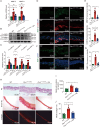

References
-
- Lacro R.V., Dietz H.C., Mahony L. Atenolol versus losartan in Marfan's syndrome. N. Engl. J. Med. 2015;372:980–981. - PubMed
-
- Forteza A., Evangelista A., Sánchez V., et al. Efficacy of losartan vs. atenolol for the prevention of aortic dilation in marfan syndrome: A randomized clinical trial. Eur. Heart J. 2016;37:978–985. - PubMed
-
- Bossone E., Eagle K.A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 2021;18:331–348. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

