This is a preprint.
PRMT5 inhibition sensitizes glioblastoma tumor models to temozolomide
- PMID: 39989968
- PMCID: PMC11844640
- DOI: 10.21203/rs.3.rs-5936706/v1
PRMT5 inhibition sensitizes glioblastoma tumor models to temozolomide
Abstract
Background: Despite multi-model therapy of maximal surgical resection, radiation, chemotherapy, and tumor-treating fields, glioblastoma patients show dismal prognosis. Protein Arginine Methyltransferase 5 (PRMT5) is overexpressed in glioblastoma and its inhibition imparts an anti-tumor effect. Even though Temozolomide (TMZ) is the standard chemotherapeutic agent in the treatment of glioblastoma, tumor cells invariably develop resistance to TMZ. However, the mechanistic role of PRMT5 in glioblastoma therapy resistance is unknown.
Methods: Patient-derived primary glioblastoma neurospheres (GBMNS), treated with PRMT5 inhibitor (LLY-283) or transfected with PRMT5 target-specific siRNA were treated with TMZ and subjected to in vitro functional and mechanistic studies. The intracranial mouse xenograft model was used to test the in vivo antitumor efficacy of combination treatment.
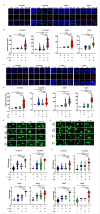
Results: We found that PRMT5 inhibition increased the cytotoxic effect and caspase 3/7 activity of TMZ in GBMNS suggesting that apoptosis is the potential mode of cell death in the combination treatment. PRMT5 inhibition abrogated the TMZ-induced G2/M cell cycle arrest. Unbiased transcriptomic studies indicate that PRMT5 inhibition negatively enriches DNA damage repair genes. Importantly, combination therapy increased DNA double-strand breaks (H2AX foci) and enhanced the DNA damage (comet assay), suggesting that the combination treatment increases the TMZ-induced DNA damage. Specifically, the LLY-283 treatment blocked homologous recombination repair in GBMNS. In vivo, LLY-283 and TMZ combination significantly curbed the tumor growth and prolonged the survival of tumor-bearing mice.
Conclusion: Concomitant treatment of LLY-283 and TMZ has significantly greater antitumor efficacy, suggesting that PRMT5 inhibition and TMZ combination could be a new therapeutic strategy for glioblastoma.
Keywords: DNA damage repair; Glioblastoma; LLY283; PRMT5; TMZ.
Conflict of interest statement
Competing interests: No, I declare that the authors have no competing interests as defi ned by BMC, orother interests that might be perceived to infl uence the results and/or discussion reported in this paper.
Figures





References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. - PubMed
-
- Qazi MA, Vora P, Venugopal C, Sidhu SS, Moffat J, Swanton C, et al. Intratumoral heterogeneity: pathways to treatment resistance and relapse in human glioblastoma. Annals oncology: official J Eur Soc Med Oncol. 2017;28(7):1448–56. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

