Personalized Therapy in a Patient With EGFR-Mutated NSCLC Developing Sequential CCDC6-RET Fusion and BRAF V600E Mutation as Bypass Resistance Mechanisms
- PMID: 39990136
- PMCID: PMC11847067
- DOI: 10.1016/j.jtocrr.2024.100773
Personalized Therapy in a Patient With EGFR-Mutated NSCLC Developing Sequential CCDC6-RET Fusion and BRAF V600E Mutation as Bypass Resistance Mechanisms
Abstract
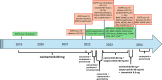
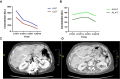
In this case report, we describe a case of sequential acquired CCDC6 -RET fusion and BRAF V600E mutation observed in a patient with EGFR-mutated NSCLC treated with osimertinib and with combined selpercatinib and osimertinib. The discovery of genomic resistance mechanisms was facilitated by serial liquid and tissue biopsies and molecular tumor board discussion. After the identification of CCDC6-RET fusion, the patient received a combination of selpercatinib and osimertinib with prolonged benefit and manageable toxicity. When a novel BRAF V600E mutation was detected at progression, the molecular tumor board suggested the administration of triple therapy, adding trametinib (anti-MEK). Nevertheless, treatment was discontinued for toxicity, highlighting the challenges of using multiple drug combinations to address complex resistance.
Keywords: BRAF V600E; Case report; EGFR-mutated NSCLC; RET fusion; Selpercatinib; Trametinib.
© 2025 Published by Elsevier Inc. on behalf of the International Association for the Study of Lung Cancer.
Conflict of interest statement
Dr. Lavaud declares receiving grants from Servier, consulting fees from Astellas, Astra Zeneca, Sanofi, and BMS, and support for attending meetings/travels from IPSEN, JANSSEN, Astellas, Pfizer, Chugai, and Sanofi. Dr. Friboulet declares receiving grants from Debiopharm, Incyte, Relay Therapeutics, Sanofi, and Nuvalent. Dr. Zalcman declares receiving honoraria from AstraZeneca, Inventiva, BMS, and Pfizer, and support for attending meetings/travels from Roche, BMS, Pfizer, Abbvie, Pharmamar, and AstraZeneca. Dr. Italiano declares receiving grants from Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono, consulting fees from Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly, honoraria from Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, IPSEN, other intellectual property interests from BMS. Dr. Besse declares receiving funding for sponsored Research from Abbvie, Amgen, AstraZeneca, Biogen, Blueprint Medicines, BMS, Celgene, Eli Lilly, GSK, Ignyta, IPSEN, Merck KGaA, MSD, Nektar, Onxeo, Pfizer, Pharma Mar, Sanofi, Spectrum Pharmaceuticals, Takeda, Tiziana Pharma, Nerviano, GSK, Pfizer, Roche-Genentech, Lilly, OSE Pharma, MSD, Celgene, Stemcentrx, Ignyta, Abbvie, Loxo Oncology, AstraZeneca, and Blueprint Medicines. Dr. Aldea declares receiving grants from Amgen, AstraZeneca, and Sandoz, and consulting fees from Viatris. The remaining authors declare no conflict of interest.
Figures
References
-
- Rehman M., Kim C., Reuss J.E., Kiedrowski L.A., Garg R.J., Liu S.V. Divergent RET- and BRAF-mediated resistance to osimertinib in EGFR-mutant NSCLC: a case report. JCO Precis Oncol. 2021;5:939–942. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

