Collagen-targeted PET imaging for progressive experimental lung fibrosis quantification and monitoring of efficacy of anti-fibrotic therapies
- PMID: 39990206
- PMCID: PMC11840721
- DOI: 10.7150/thno.106367
Collagen-targeted PET imaging for progressive experimental lung fibrosis quantification and monitoring of efficacy of anti-fibrotic therapies
Abstract
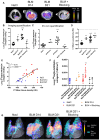
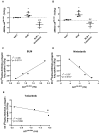
Idiopathic pulmonary fibrosis (IPF) is a progressive disease characterized by an excessive collagen deposition ultimately leading to tissue stiffening and functional decline. Beyond IPF, other progressive pulmonary fibrosis are often associated with connective tissue diseases and may develop in ∼18-32% of patients. Therapeutic options are limited to nintedanib and pirfenidone which are only able to reduce fibrosis progression without curing it. The current lack of biomarker to accurately assess and predict disease progression and therapy efficacy for IPF remains a major clinical concern. Methods: In our study, collagen deposition was monitored in bleomycin-induced lung fibrosis in mice by in vivo molecular imaging using a collagen-targeted radiopharmaceutical, [68Ga]Ga-NODAGA-collagelin. Fibrosis progression was also monitored using computed tomography, the gold standard technique to detect lung fibrosis in patients. Results: We demonstrated that the bleomycin-induced increase in collagen lung content can be accurately quantified by [68Ga]Ga-NODAGA-collagelin PET imaging in correlation with disease stage and severity. The lung uptake of [68Ga]Ga-NODAGA-collagelin was mainly found in fibrotic areas of lungs in bleomycin-receiving mice. Most interestingly, [68Ga]Ga-NODAGA-collagelin PET imaging allowed the in vivo non-invasive monitoring of nintedanib efficacy as well as the anti-fibrotic effect of the JAK inhibitor, tofacitinib. Conclusion: Thus, collagen-targeted PET imaging appears as a promising non-invasive tool for staging, monitoring and prediction of disease progression and therapy efficacy towards personalized medicine in IPF.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Wijsenbeek M, Cottin V. Spectrum of Fibrotic Lung Diseases. N Engl J Med. 2020;383:958–68. - PubMed
-
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ. et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. - PubMed
-
- Gupta R, Morgan AD, George PM, Quint JK. Incidence, prevalence and mortality of idiopathic pulmonary fibrosis in England from 2008 to 2018: a cohort study. Thorax. 2024;79:624–31. - PubMed
-
- Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J. et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192:e3–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

