This is a preprint.
Bacterial pathogen deploys iminosugar galactosyrin to manipulate plant glycobiology
- PMID: 39990308
- PMCID: PMC11844564
- DOI: 10.1101/2025.02.13.638044
Bacterial pathogen deploys iminosugar galactosyrin to manipulate plant glycobiology
Update in
-
Bacterial pathogen deploys the iminosugar glycosyrin to manipulate plant glycobiology.Science. 2025 Apr 18;388(6744):297-303. doi: 10.1126/science.adp2433. Epub 2025 Apr 17. Science. 2025. PMID: 40245141 Free PMC article.
Abstract
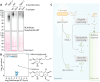
The extracellular space (apoplast) of plants is an important molecular battleground during infection by many pathogens. We previously found that a plant-secreted β-galactosidase BGAL1 acts in immunity by facilitating the release of immunogenic peptides from bacterial flagellin and that Pseudomonas syringae suppresses this enzyme by producing a small molecule inhibitor called galactosyrin. Here, we elucidated the structure and biosynthesis of galactosyrin and uncovered its multifunctional roles during infection. Structural elucidation by cryo-EM and chemical synthesis revealed that galactosyrin is an iminosugar featuring a unique geminal diol attached to the pyrrolidine moiety that mimics galactose binding to the β-galactosidase active site. Galactosyrin biosynthesis branches off from purine biosynthesis and involves three enzymes of which the first is a reductase that is unique in iminosugar biosynthesis. Besides inhibiting BGAL1 to avoid detection, galactosyrin also changes the glycoproteome and metabolome of the apoplast. The manipulation of host glycobiology may be common to plant-associated bacteria that carry putative iminosugar biosynthesis clusters.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Doehlemann G., Hemetsberger C., Apoplastic immunity and its suppression by filamentous plant pathogens. New Phytol. 198, 1001–1016 (2013). - PubMed
-
- Buscaill P., Chandrasekar B., Sanguankiattichai N., Kourelis J., Kaschani F., Thomas E. L., Morimoto K., Kaiser M., Preston G. M., Ichinose Y., van der Hoorn R. A. L., Glycosidase and glycan polymorphism control hydrolytic release of immunogenic flagellin peptides. Science (80-.). 364 (2019). - PubMed
-
- Wei C., Kvitko B. H., Shimizu R., Crabill E., Alfano J. R., Lin N., Martin G. B., Huang H., Collmer A., A Pseudomonas syringae pv. tomato DC3000 mutant lacking the type III effector HopQ1–1 is able to cause disease in the model plant Nicotiana benthamiana. Plant J. 51, 32–46 (2007). - PubMed
-
- Vencato M., Tian F., Alfano J. R., Buell C. R., Cartinhour S., DeClerck G. A., Guttman D. S., Stavrinides J., Joardar V., Lindeberg M., Bronstein P. A., Mansfield J. W., Myers C. R., Collmer A., Schneider D. J., Bioinformatics-enabled identification of the HrpL regulon and type III secretion system effector proteins of Pseudomonas syringae pv. phaseolicola 1448A. Mol. Plant-Microbe Interact. 19, 1193–1206 (2006). - PubMed
-
- Xie Y., Shao X., Deng X., Regulation of type III secretion system in Pseudomonas syringae. Environ. Microbiol. 21, 4465–4477 (2019). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
