This is a preprint.
Genomic and functional adaptations in guanylate-binding protein 5 (GBP5) highlight specificities of bat antiviral innate immunity
- PMID: 39990348
- PMCID: PMC11844482
- DOI: 10.1101/2025.02.11.637683
Genomic and functional adaptations in guanylate-binding protein 5 (GBP5) highlight specificities of bat antiviral innate immunity
Abstract
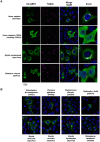
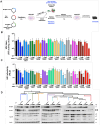
Bats are asymptomatic reservoirs of several zoonotic viruses. This may result from long-term coevolution between viruses and bats, that have led to host adaptations contributing to an effective balance between strong antiviral responses with innate immune tolerance. To better understand these virus-host interactions, we combined comparative transcriptomics, phylogenomics and functional assays to characterize the evolution of bat innate immune antiviral factors. First, we stimulated the type I interferon immune pathway in Myotis yumanensis primary cells and identified guanylate-binding protein 5 (GBP5) as the most differentially expressed interferon-stimulated gene (ISG). Phylogenomic analyses showed that bat GBP5 has been under strong episodic positive selection, with numerous rapidly evolving sites and species-specific gene duplications, suggesting past evolutionary arms races. Functional tests on GBP5 orthologs from ten bat species covering the >60 million years of Chiroptera evolution revealed species- and virus-specific restrictions against RNA viruses (retrovirus HIV, and rhabdoviruses European bat lyssavirus and VSV), which are typical signatures of adaptations to past viral epidemics. Interestingly, we also observed a lineage-specific loss of the GBP5 prenylation motif in the common ancestor of Pipistrellus and Eptesicus bats, associated with different GBP5 subcellular localization and loss of antiviral functions. Resurrection of the ancestral prenylation motif in Eptesicus fuscus GBP5 rescued its subcellular localization, but not the complete antiviral activities, suggesting that additional determinants are necessary for the antiviral restriction. Altogether, our results highlight adaptations that contribute to bat specific immunity and provide insights into the functional evolution of antiviral effector GBP5.
Keywords: Chiroptera; EBLV-1; GBP5; Guanylate-binding proteins; HIV; Rhabdovirus; antiviral factor; bats; innate immunity; molecular arms-race; positive selection; retrovirus; virus-host coevolution.
Figures





References
-
- Tenthorey J. L., Emerman M. & Malik H. S. Evolutionary Landscapes of Host-Virus Arms Races. Annu. Rev. Immunol. 40, 271–294 (2022). - PubMed
-
- Irving A. T., Ahn M., Goh G., Anderson D. E. & Wang L.-F. Lessons from the host defences of bats, a unique viral reservoir. Nature 589, 363–370 (2021). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
