This is a preprint.
A Massively Parallel CRISPR-Based Screening Platform for Modifiers of Neuronal Activity
- PMID: 39990495
- PMCID: PMC11844385
- DOI: 10.1101/2024.02.28.582546
A Massively Parallel CRISPR-Based Screening Platform for Modifiers of Neuronal Activity
Abstract
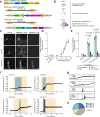
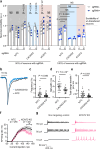
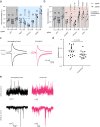
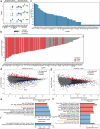
Understanding the complex interplay between gene expression and neuronal activity is crucial for unraveling the molecular mechanisms underlying cognitive function and neurological disorders. Here, we developed pooled screens for neuronal activity, using CRISPR interference (CRISPRi) and the fluorescent calcium integrator CaMPARI2. Using this screening method, we evaluated 1343 genes for their effect on excitability in human iPSC-derived neurons, revealing potential links to neurodegenerative and neurodevelopmental disorders. These genes include known regulators of neuronal excitability, such as TARPs and ion channels, as well as genes associated with autism spectrum disorder and Alzheimer's disease not previously described to affect neuronal excitability. This CRISPRi-based screening platform offers a versatile tool to uncover molecular mechanisms controlling neuronal activity in health and disease.
Conflict of interest statement
M.K. is a co-scientific founder of Montara Therapeutics and serves on the Scientific Advisory Boards of Engine Biosciences, Casma Therapeutics, Alector, and Montara Therapeutics, and is an advisor to Modulo Bio and Recursion Therapeutics. M.K. is an inventor on US Patent 11,254,933 related to CRISPRi and CRISPRa screening, and on a US Patent application on in vivo screening methods. M.T., X.H., A.C, C.G.J., C.E., and J.H. are employees of Genentech. S.C.B is currently employed at the Arc Institute (Palo Alto, CA).
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
