Febuxostat therapy improved the outcomes of cardiorenal syndrome rodent through alleviating xanthine oxidase-induced oxidative stress and mitochondrial dysfunction
- PMID: 39990671
- PMCID: PMC11844282
- DOI: 10.7150/ijbs.99194
Febuxostat therapy improved the outcomes of cardiorenal syndrome rodent through alleviating xanthine oxidase-induced oxidative stress and mitochondrial dysfunction
Abstract
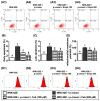
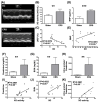
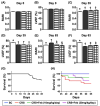
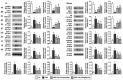
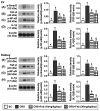
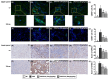
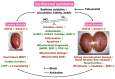
Background: We tested the hypothesis that febuxostat (Feb) therapy effectively protected cardiorenal syndrome (CRS) rats via repressing the xanthine-oxidase (XO)-caused oxidative stress. Methods and Results: Cellular levels of apoptosis/oxidative stress/mitochondrial-membrane potential were higher in p-Cresol treated-NRK-52E cells than in control group that were reversed by Feb treatment or silencing XO gene (all P<0.001). Pilot study demonstrated that: XO activity was significantly increased in CRS than in SC group; a significant negative correlation between XO activity and left ventricular ejection fraction (LVEF) (%); a significant positive correlation between XO activity and BNP/BUN/creatinine/proteinuria levels (all P<0.01). Male-adult SD-rats were classified into groups 1(sham-control)/2 (CRS)/3 [CRS+Feb (10mg/kg/day)]/4 [CRS+Feb (30mg/kg/day)]. By day-63, the survival rate was significantly lower in group 2 than in other groups (P=0.029), and circulatory levels of FGF23/BNP/XO-activity BUN/creatinine/proteinuria and renal-artery resistance were highest in group 2/lowest in group 1/significantly lower in group 4 than in group 3, whereas the LVEF exhibited an opposite pattern of XO among the groups (all P<0.0001). Cellular levels of fibrosis/XO/H2DCFDA/CD68/CHAC1, and protein expressions of oxidative-stress (NOX-2/NOX-4/XO)/inflammatory (NF-κB/IL-1β)/fibrotic (Smad3/TFG-β)/apoptotic (CHAC1/2)/mitochondrial-damaged (p-DRP1) biomarkers in kidney/heart tissues displayed a similar pattern of XO (all P<0.0001). Conclusion: Feb therapy improved cardiorenal function and prognostic outcome in CRS rats.
Keywords: apoptosis; inflammation; mitochondrial biogenesis; oxidative stress; xanthine oxidase.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39. - PubMed
-
- Ronco C, Bellasi A, Di Lullo L. Cardiorenal Syndrome: An Overview. Adv Chronic Kidney Dis. 2018;25:382–90. - PubMed
-
- Zannad F, Rossignol P. Cardiorenal Syndrome Revisited. Circulation. 2018;138:929–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

