Prolonged DEHP exposure enhances the stemness and metastatic potential of TNBC cells in an MSI2-dependent manner
- PMID: 39990676
- PMCID: PMC11844279
- DOI: 10.7150/ijbs.101598
Prolonged DEHP exposure enhances the stemness and metastatic potential of TNBC cells in an MSI2-dependent manner
Abstract
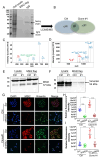
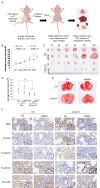
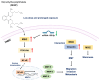
Di(2-ethylhexyl) phthalate (DEHP) is a commonly used plasticizer, and human exposure to phthalates is a major health concern. DEHP, which is widely recognized as an endocrine disruptor, is associated with an increased risk of several diseases, including breast cancer. Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer, and metastasis is the leading cause of TNBC-related mortality. However, the correlation between DEHP exposure and TNBC metastasis remains elusive. In the present study, we found that prolonged DEHP treatment enhanced the migration and invasion of TNBC cells both in vitro and in vivo. Mechanistically, DEHP exposure induced Musashi RNA binding protein 2 (MSI2) overexpression, which subsequently activated the PI3K/Akt/NF-κB/MMP-9 axis to augment metastatic potential. MSI2 also promoted stemness. Interestingly, we identified a novel function of MSI2 in regulating the expression, distribution, and polarization of vimentin that is independent of its conventional RNA binding and translation regulation. Genetic knockdown of MSI2 potently abolished DEHP-mediated TNBC progression. Moreover, MSI2 depletion inhibited lung metastasis in metastatic mouse models but did not affect proliferation or tumor size. Intriguingly, miR-155-5p downregulation was observed after DEHP exposure, while mimic miR-155-5p treatment inhibited DEHP-induced TNBC migration, accompanied by reduced expression of MSI2 and vimentin. These findings suggested an inverse relationship between miR-155-5p levels and MSI2 expression. Taken together, MSI2 might serve as a potential therapeutic target and function as a prognostic biomarker for TNBC patients.
Keywords: Di-2-ethylhexyl phthalate (DEHP); Metastasis; Musashi RNA binding protein 2 (MSI2); Stemness; Triple-negative breast cancer (TNBC); miR-155-5p.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures







References
-
- Api AM. Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food Chem Toxicol. 2001;39:97–108. - PubMed
-
- Li PC, Li XN, Du ZH, Wang H, Yu ZR, Li JL. Di (2-ethyl hexyl) phthalate (DEHP)-induced kidney injury in quail (Coturnix japonica) via inhibiting HSF1/HSF3-dependent heat shock response. Chemosphere. 2018;209:981–8. - PubMed
-
- Kaur J, Mania I. Deep transcranial magnetic stimulation (dTMS) for treatment of major depressive disorder (MDD) status post-surgical removal of medulloblastoma: A case report of safety. Brain Stimul. 2019;12:1061–2. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

