Iptacopan Reduces Proteinuria and Stabilizes Kidney Function in C3 Glomerulopathy
- PMID: 39990880
- PMCID: PMC11843281
- DOI: 10.1016/j.ekir.2024.10.023
Iptacopan Reduces Proteinuria and Stabilizes Kidney Function in C3 Glomerulopathy
Abstract
Introduction: C3 glomerulopathy (C3G) is a complex, chronic, ultra rare, progressive primary glomerulonephritis, resulting from alternative complement pathway overactivation, leading to kidney failure in most patients, and frequent recurrence in transplants. Iptacopan (LNP023) is an oral, proximal complement inhibitor specifically targeting factor B, that selectively inhibits the alternative complement pathway.
Methods: This was a phase 2 extension study of 26 adult patients with native kidney (cohort A), or recurrent C3G (post kidney transplantation; cohort B) receiving open label iptacopan.
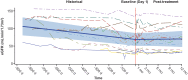
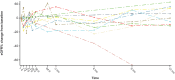
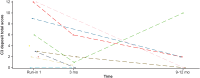
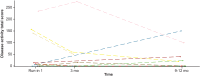
Results: At 12 months, patients in cohort A had a significant reduction in 24-hour urine protein-to-creatinine ratio (UPCR; 57%; P < 0.0001; confidence interval [CI]: 0.31-0.59), an improvement in estimated glomerular filtration rate (eGFR; 6.83 ml/min per 1.73 m2; P = 0.0174; CI: 1.25-12.40), and an increase in serum C3 levels (geometric mean ratio to baseline: 3.53; P < 0.0001; CI: 3.01-4.15). In cohort B, most patients had normal urinary protein excretion at baseline (mean [range] 24-hour UPCR: 121 [9-445]), which was slightly lower by 12 months (21% reduction; CI: 0.48-1.31; P = 0.3151). In cohort B at 12 months, mean eGFR was at baseline values (mean change from baseline: -0.96 ml/min per 1.73 m2; P = 0.7335; CI: -6.60 to 4.69). Cohort B patients had significantly higher serum C3 values at 12 months compared with baseline (ratio:1.96; CI: 1.70-2.27; P < 0.0001). In cohorts A + B combined, the median difference in C3 deposit score on renal biopsy from baseline was -7.00 (CI: -12.00 to 4.00;) at 9 to 12 months treatment with iptacopan.
Conclusion: These data provide a clinical rationale for further evaluation of long-term treatment of C3G with iptacopan.
Keywords: C3 glomerulopathy; C3G; complement pathway; iptacopan; kidney; transplantation.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

