Controllable intein splicing and N-terminal cleavage at mesophilic temperatures
- PMID: 39991137
- PMCID: PMC11842431
- DOI: 10.3389/fbioe.2025.1543573
Controllable intein splicing and N-terminal cleavage at mesophilic temperatures
Abstract
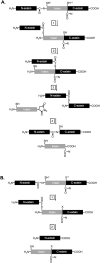
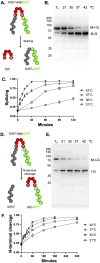
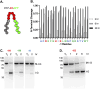
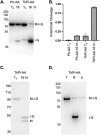
Inteins (intervening proteins) interrupt host proteins and are removed through a protein splicing reaction that ligates adjacent N- and C-exteins. The ability of inteins to specifically rearrange peptide bonds has proven exceptionally useful in protein engineering, thus, methods to control intein activity are of considerable interest. One particularly useful application of inteins is for the removal of an affinity tag following purification of a target protein through N-terminal cleavage (NTC). Typically, extended incubation at high temperature (greater than 50°C) or with an external nucleophile (e.g., dithiothreitol) is required to drive NTC, conditions that compromise the folding of many target proteins. Here, we characterize a variant of the Thermococcus kodakarensis RadA intein that can perform NTC at moderate temperatures in the absence of an external nucleophile. While we find that while NTC is largely inhibited during expression in Escherichia coli at 15°C, rapid and efficient NTC can be activated 37°C. Our results provide an alternative intein-based system - one that does not require either an external nucleophile or prolonged incubation at high temperature to stimulate NTC - that controls intein activity within a temperature range amenable to most mesophilic experimental organisms.
Keywords: N-terminal cleavage; biosensor; bioseparations; intein; protein splicing.
Copyright © 2025 McNeal, Weinberger, Liman, Ariagno, Wood, Santangelo and Lennon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

