Modulation of cardiomyocyte contractility and action potentials with chemogenetic chloride currents
- PMID: 39992007
- PMCID: PMC11908473
- DOI: 10.1113/JP286428
Modulation of cardiomyocyte contractility and action potentials with chemogenetic chloride currents
Abstract
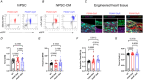
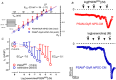
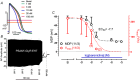
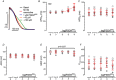
Transient perturbation of electrical activity is used in neuroscience to study the impact of specific neuronal cell populations on brain function. Similarly, cardiomyocyte (CM) physiology can be controlled by the activation of artificially expressed ion channels. Pharmacologically selective actuator modules (PSAMs) are engineered ligand-gated ion channels that can be activated with small molecules. We aimed to use the 'inhibitory' PSAMs, (i) PSAML141F,Y115F-GlyR (PSAM-GlyR) and (ii) PSAML131G,Q139L,Y217F (ultrapotent PSAM4-GlyR), which consist of modified α7-nicotinergic acetylcholine receptor ligand binding domains and the ion pore domain of the glycine receptor, to modulate CM physiology with chloride currents. We employed CRISPR/Cas9 to integrate PSAM-GlyR and PSAM4-GlyR in induced pluripotent stem cells, differentiated CMs and generated engineered heart tissue (EHT). Video optical force recordings, sharp microelectrode action potential measurements and patch-clamp technique were used to characterize PSAM-GlyR and PSAM4-GlyR CMs. PSAM-GlyR and PSAM4-GlyR activation allowed titration of chloride currents in a reversible manner. We found that chloride currents modulated action potential characteristics. Patch clamp recordings showed that channel activation resulted in chloride-driven currents that depolarized the cell. In EHT, this resulted in a stop of contractility that was fully reversible after wash-out. We provide a comprehensive characterization of the chemogenetic tools PSAM-GlyR and PSAM4-GlyR in CMs, demonstrating their utility to modulate CM activity in vitro (PSAM-GlyR and PSAM4-GlyR) but also potential for in vivo applications (PSAM4-GlyR). KEY POINTS: Pharmacologically selective actuator modules (PSAMs) are engineered ligand-gated ion channels that can be activated with small molecules. These chemogenetic tools have been applied in neuroscience to inhibit neuronal activity. Chemogenetic tools can also be used to modulate cardiomyocyte physiology. Activation of the PSAMs, PSAM-GlyR and PSAM4-GlyR depolarized cardiomyocytes and thus stopped cardiac contractility. Our study characterizes novel tools that can be used to modulate cardiomyocyte physiology in vitro and in vivo.
Keywords: cardiac electrophysiology; cardiac function; cardiomyocyte; chemogenetics; pluripotent stem cells.
© 2025 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors have no competing interests.
Figures






References
-
- Armstrong, L. C. , Kirsch, G. E. , Fedorov, N. B. , Wu, C. , Kuryshev, Y. A. , Sewell, A. L. , Liu, Z. , Motter, A. L. , Leggett, C. S. , & Orr, M. S. (2017). High‐throughput patch clamp screening in human α6‐containing nicotinic acetylcholine receptors. Society for Laboratory Automation and Screening Discovery, 22(6), 686–695. - PMC - PubMed
-
- Breckwoldt, K. , Letuffe‐Brenière, D. , Mannhardt, I. , Schulze, T. , Ulmer, B. , Werner, T. , Benzin, A. , Klampe, B. , Reinsch, M. C. , Laufer, S. , Shibamiya, A. , Prondzynski, M. , Mearini, G. , Schade, D. , Fuchs, S. , Neuber, C. , Krämer, E. , Saleem, U. , Schulze, M. L. , … Hansen, A. (2017). Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nature Protocols, 12(6), 1177–1197. - PubMed
-
- Das, P. , & Dillon, G. H. (2005). Molecular determinants of picrotoxin inhibition of 5‐hydroxytryptamine type 3 receptors. Journal of Pharmacology and Experimental Therapeutics, 314(1), 320–328. - PubMed
-
- Eschenhagen, T. , & Weinberger, F. (2024). Challenges and perspectives of heart repair with pluripotent stem cell‐derived cardiomyocytes. Nature Cardiovascular Research, 3(5), 515–524. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
