MCAM is a prognostic biomarker in patients with liver cirrhosis and HCC
- PMID: 39992088
- PMCID: PMC11458167
- DOI: 10.1097/HC9.0000000000000532
MCAM is a prognostic biomarker in patients with liver cirrhosis and HCC
Abstract
Background: Despite the rising prevalence of liver cirrhosis and HCC worldwide, reliable prognostic blood biomarkers are lacking. Melanoma cell adhesion molecule (MCAM) is a cell adhesion protein, and its cleavage by metalloproteinases, known to be enriched in fibrotic and malignant diseases, results in the release of a soluble form into the blood. The aim of this study was to characterize MCAM expression in patients with chronic liver disease and to evaluate soluble MCAM (sMCAM) as a prognostic blood biomarker in patients with liver cirrhosis and HCC.
Methods: Expression of MCAM in liver tissue was assessed using transcriptomic data sets as well as by immunohistochemical analyses in patients with liver cirrhosis and HCC. Moreover, sMCAM blood levels were determined in plasma samples from healthy controls (n = 8), patients with chronic liver disease (n = 66), liver cirrhosis (n = 236), and HCC (n = 72).
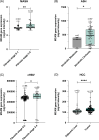
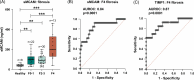
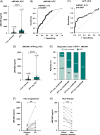
Results: Single-cell RNA sequencing and immunohistochemistry indicated MCAM to be highly expressed by liver endothelial cells and fibroblasts. Expression was upregulated in liver tissue of patients with liver fibrosis and especially HCC independent of the underlying etiology (p < 0.05, respectively). Blood levels of sMCAM increased with fibrosis stage and peaked in patients with concomitant HCC, showing a comparable diagnostic performance as the fibrosis markers hyaluronic acid (HA) and TIMP1 for diagnosis of liver cirrhosis (AUROCsMCAM = 0.84, AUROCHA = 0.89, AUROCTIMP1 = 0.87) and as alpha-fetoprotein (AFP) for diagnosis of HCC (AUROCsMCAM = 0.72, AUROCAFP = 0.72). Finally, high sMCAM levels predicted worse survival in HCC (p < 0.001).
Conclusions: Collectively, our study suggests sMCAM as a blood biomarker of a liver microenvironment that drives the progression of liver disease in patients with liver cirrhosis and HCC.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Dominik Bettinger is on the speaker’s bureau for Gore and Falk Foundation. He received grants from Abbvie. Simon Johannes Garing received grants from Ipsen, Gilead, and Schwiete Foundation. Friedrich Foerster advises and is on the speaker’s bureau for AstraZeneca and Roche. He advises BMS and Eisai. He is on the speakers’ bureau for MSD and Pfizer. He received grants from Merck KGaA and Servier. Bertram Bengsch advises for Roche. The remaining authors have no conflicts to report.
Figures


References
-
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. - PubMed
-
- Ascione A, Fontanella L, Imparato M, Rinaldi L, de Luca M. Mortality from cirrhosis and hepatocellular carcinoma in Western Europe over the last 40 years. Liver Int. 2017;37:1193–1201. - PubMed
-
- European Association for the Study of the Liver . Electronic address: easloffice@easloffice.eu, Clinical Practice Guideline Panel, Chair:, EASL Governing Board representative:, Panel members: EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J Hepatol. 2021;75:659–689. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

