Usefulness of a hub and spoke TDM-guided expert clinical pharmacological advice program of dalbavancin for optimizing very long-term curative or suppressive treatment of chronic staphylococcal infections
- PMID: 39992102
- PMCID: PMC11963596
- DOI: 10.1128/aac.01830-24
Usefulness of a hub and spoke TDM-guided expert clinical pharmacological advice program of dalbavancin for optimizing very long-term curative or suppressive treatment of chronic staphylococcal infections
Abstract
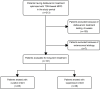
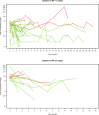
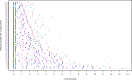
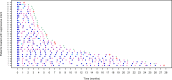
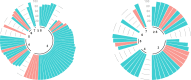
A hub and spoke model for optimizing long-term treatment of chronic staphylococcal infections with dalbavancin based on therapeutic drug monitoring (TDM)-guided expert clinical pharmacological advice (ECPA) was implemented. This multicentric retrospective cohort study included patients receiving dalbavancin monotherapy lasting >6 weeks at different spoke hospitals having treatment optimized by means of a TDM-guided ECPA program at a hub hospital. Optimal pharmacokinetic/pharmacodynamic target against staphylococci with an MIC up to 0.125 mg/L was defined as dalbavancin concentrations >8.04 mg/L. Patients received dalbavancin therapy for curative (curative group) or suppressive (suppressive group) purposes. Clinical outcome was assessed by means of repeated ambulatory visits. A total of 12 spoke hospitals applied for 414 TDM-based ECPA for 101 patients, of whom 64.4% (65/101) were treated for curative and 35.6% (36/101) were for suppressive purposes. In the curative and suppressive groups, TDM-based ECPA optimized treatment for up to 14 and 28 months, respectively, and ensured median optimal exposure of 95.7% and 100%, respectively. In the curative group, having <70% of treatment time with concentrations above the optimal target increased failure risk [odds ratio (OR), 6.71; confidence interval (CI), 0.97-43.3; P = 0.05]. In the suppressive group, infective endocarditis was associated with an increased risk of ineffective treatment (OR, 8.65; CI, 1.29-57.62; P = 0.046). Mild adverse events were reported in 4.5% (5/101) of cases. A hub and spoke TDM-guided ECPA program of dalbavancin may be cost-effective for optimizing long-term treatment of chronic staphylococcal infections and for patients admitted to hospitals lacking in-house MD clinical pharmacologists.
Keywords: dalbavancin; long-term treatment; model-informed precision dosing; therapeutic drug monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

