Evoked delayed potential ablation for post-myocardial infarction ventricular tachycardia: results from a large prospective multicentre study
- PMID: 39992165
- PMCID: PMC11848844
- DOI: 10.1093/europace/euaf003
Evoked delayed potential ablation for post-myocardial infarction ventricular tachycardia: results from a large prospective multicentre study
Abstract
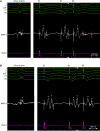
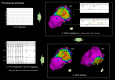
Aims: The optimal substrate ablation approach for post-myocardial infarction (MI) ventricular tachycardia (VT) is unknown. Proposed ablation targets are prone to individual interpretation making the ablation outcome potentially operator dependent. Evoked delayed potentials (EDPs) are a well-defined target. Evoked delayed potential ablation was effective in preventing post-MI VT recurrence in a prior study. The aims of this study were to assess long-term outcomes of EDP ablation in a large multicentre cohort of post-MI patients and to compare ablation outcomes between centres with and without prior experience in EDP ablation.
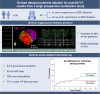
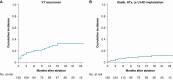
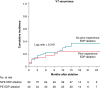
Methods and results: Patients with post-MI VT undergoing ablation in one centre performing EDP ablation since 2013 and five centres without prior experience in EDP ablation were prospectively included. A uniform mapping protocol including right ventricular extra-stimulation aiming to EDP identification was followed. Ablation endpoints were EDP elimination and VT non-inducibility. Patients were followed for VT recurrence, mortality, heart transplant, and left ventricular assist device implantation. In total, 130 patients were included. The protocol was successfully performed in 99%, and in 94%, EDPs were identified and ablated. In total, 78% of patients were rendered non-inducible. Ventricular tachycardia-free survival was 78% [95% confidence interval (CI) 71-85] and 71% (95% CI 63-80) at 6 and 12 months, respectively. No difference in VT-free survival was observed among centres with and without prior experience in EPD ablation.
Conclusion: In a large multicentre prospective cohort of patients with post-MI VT, EDP ablation resulted in good long-term outcomes. Importantly, VT recurrence rates did not differ among centres with and without prior experience in EDP ablation, indicating that this approach can be easily reproduced by operators previously not familiar with the technique.
Keywords: Evoked delayed potentials; Functional substrate mapping; Myocardial infarction; Substrate ablation; Substrate modification; Ventricular tachycardia.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: The Department of Cardiology Leiden received unrestricted research and fellowship grants from Edward Lifesciences, Boston Scientific, Medtronic, Biotronik, and Biosense Webster. A.O. received consulting fees from Abbott, Biosense Webster, Boston Scientific, and Medtronic. K.V. received research grants from Medtronic, Biosense Webster and Abbott, and consulting fees from Medtronic, Abbott, Biosense Webster, Philips, Boston Scientific and AstraZeneca; all paid to the Cardiovascular Research Institute of Maastricht. None of the other authors declared a conflict of interest.
Figures

References
-
- Di Biase L, Burkhardt JD, Lakkireddy D, Carbucicchio C, Mohanty S, Mohanty P et al. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy: the VISTA randomized multicenter trial. J Am Coll Cardiol 2015;66:2872–82. - PubMed
-
- Silberbauer J, Oloriz T, Maccabelli G, Tsiachris D, Baratto F, Vergara P et al. Noninducibility and late potential abolition: a novel combined prognostic procedural end point for catheter ablation of postinfarction ventricular tachycardia. Circ Arrhythm Electrophysiol 2014;7:424–35. - PubMed
-
- Jaïs P, Maury P, Khairy P, Sacher F, Nault I, Komatsu Y et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation 2012;125:2184–96. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

