Long-term outcomes of volume de-escalation for breast nodal irradiation
- PMID: 39992611
- PMCID: PMC12006277
- DOI: 10.1007/s10549-025-07652-3
Long-term outcomes of volume de-escalation for breast nodal irradiation
Abstract
Introduction: NCCN recommendations suggest irradiating chest wall/breast only + regional node irradiation (RNI) of the undissected axillary levels for node-positive breast cancer (BC) patients. We retrospectively analyzed a cohort of node-positive BC patients who received adjuvant radiotherapy (RT) with a volume de-escalation at the level of axillary nodes.
Material and methods: We conducted a retrospective analysis of node-positive BC patients treated with adjuvant RT administered following a conventional fractionation schedule using a 3D-conformal technique to the chest wall or breast and only the IV axillary level. The primary endpoint of the study was disease free survival (DFS). Secondary endpoints included loco-regional control (LRC), and Overall Survival (OS). Toxicity was documented according to the Radiation Therapy Oncology Group (RTOG) criteria.
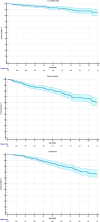
Results: A total cohort of 343 patients was analyzed. Loco-regional recurrence occurred in 100 (29.1%). The 5- and 10-year Kaplan-Meyer curves for DFS were 81.4% (95% CI: 79.3%-83.5%) and 60.9% (95% CI: 57.6%-64.5%), respectively. Multivariate Cox analysis confirmed that lymph node ratio (HR = 9.76, 95% CI: 3.12-30.53, p = 0.0001), Luminal B subtype (HR = 2.03, 95% CI: 1.26-3.29, p = 0.004), and triple-negative subtype (HR = 2.70, 95% CI: 1.22-5.99, p = 0.01) were significant predictors of poor DFS. Lymphedema in the ipsilateral arm was reported in 32 (9.3%) patients, primarily Grade 1 or 2.
Conclusions: Improved patients' selection and a broader use of systemic therapy could make de-escalation a feasible option. However, this approach should be avoided in patients with extensive nodal involvement, specific molecular subtypes, or comorbidities that prevent the use of chemotherapy.
Keywords: Breast cancer; Long-term outcomes; Regional nodal irradiation; Volume de-escalation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the IRB of the participating centers. Informed consent: Informed consent was obtained from all individual participants involved in the study.
Figures
References
-
- Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA et al (2022) Breast cancer statistics, 2022. CA Cancer J Clin 72(6):524–541. 10.3322/caac.21754 - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. 10.3322/caac.21660 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical