Intratumoral Fusobacterium nucleatum Recruits Tumor-Associated Neutrophils to Promote Gastric Cancer Progression and Immune Evasion
- PMID: 39992708
- PMCID: PMC12079103
- DOI: 10.1158/0008-5472.CAN-24-2580
Intratumoral Fusobacterium nucleatum Recruits Tumor-Associated Neutrophils to Promote Gastric Cancer Progression and Immune Evasion
Abstract
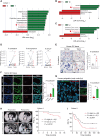
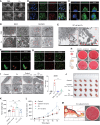
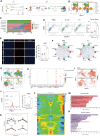
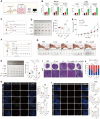
Intratumoral microbiota can affect the development and progression of many types of cancer, including gastric cancer. A better understanding of the precise mechanisms by which microbiota support gastric cancer could lead to improved therapeutic approaches. In this study, we investigated the effect of intratumoral microbiota on the tumor immune microenvironment during gastric cancer malignant progression. Analysis of human gastric cancer tissues with 16S rRNA amplicon sequencing revealed that Fusobacterium nucleatum was significantly enriched in gastric cancer tissues with lymph node metastasis and correlated with a poor prognosis. F. nucleatum infection spontaneously induced chronic gastritis and promoted gastric mucosa dysplasia in mice. Furthermore, gastric cancer cells infected with F. nucleatum showed accelerated growth in immunocompetent mice compared with immunodeficient mice. Single-cell RNA sequencing uncovered that F. nucleatum recruited tumor-associated neutrophils (TAN) to reshape the tumor immune microenvironment. Mechanistically, F. nucleatum invaded gastric cancer cells and activated IL17/NF-κB/RelB signaling, inducing TAN recruitment. F. nucleatum also stimulated TAN differentiation into the protumoral subtype and subsequent promotion of PD-L1 expression, further facilitating gastric cancer immune evasion while also enhancing the efficacy of anti-PD-L1 antibody therapy. Together, these data uncover mechanisms by which F. nucleatum affects gastric cancer immune evasion and immunotherapy efficacy, providing insights for developing effective treatment strategies. Significance: Intratumoral F. nucleatum activates NF-κB signaling to facilitate gastric cancer immune evasion by promoting tumor-associated neutrophil recruitment that sensitizes tumors to immune checkpoint blockade therapy.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
T. Zhang reports grants from the Guangdong Provincial Natural Science Foundation, the Guangdong Provincial Health Commission, and the Fundamental Research Funds for the Central Universities, Sun Yat-sen University during the conduct of the study. Y. Qian reports grants from the Guangzhou Science and Technology Programme and the Guangdong Provincial Natural Science Foundation project during the conduct of the study. J. Chen reports grants from the Guangdong Provincial Natural Science Foundation project and the National Natural Science Foundation of China during the conduct of the study. S. Cai reports grants from the National Natural Science Foundation of China and the Guangdong Provincial Natural Science Foundation during the conduct of the study. No disclosures were reported by the other authors.
Figures




References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2002;2:28–37. - PubMed
-
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1:1311–5. - PubMed
-
- Fu K, Cheung AHK, Wong CC, Liu W, Zhou Y, Wang F, et al. . Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell 2024;187:882–96.e17. - PubMed
MeSH terms
Substances
Grants and funding
- 82173239/National Natural Science Foundation of China grant
- 82472904/National Natural Science Foundation of China grant
- 82100626/National Natural Science Foundation of China grant
- 2023A1515111152/Guangdong Provincial Natural Science Foundation project grant
- 2021A1515110759/Guangdong Provincial Natural Science Foundation project grant
- 2023A1515011187/Guangdong Provincial Natural Science Foundation project grant
- 2022A1515011534/Guangdong Provincial Natural Science Foundation project grant
- 2024A1515010739/Guangdong Provincial Natural Science Foundation project grant
- 2022B1111070006/Key-Area Research and Development Program of Guangdong Province grant
- 2022GDASZH-2022010101/GDAS' Project of Science and Technology Development
- A2024052/Guangdong Provincial Health Commission grant
- 24qnpy329/The Fundamental Research Funds for the Central Universities, Sun Yat-sen University
- 2024MS006/Guangzhou Nansha Technology Plan Project
- 2023MS003/Guangzhou Nansha Technology Plan Project
- 2024B1515040029/Guangdong Province Excellent Youth Team Project
- 2022B1111070006/Guangdong Provincial Key Laboratory of Construction Foundation ()
- 2022GDASZH-2022010101/Guangdong Academy of Sciences (GDAS)
- A2024052/Guangzhou Science and Technology Innovation Center (Guangdong Provincial Modern Agricultural Science and Technology Innovation Center)
- 24qnpy329/Fundamental Research Funds for the Central Universities (Fundamental Research Fund for the Central Universities)
- 2024B1515040029/Guangdong Provincial Applied Science and Technology Research and Development Program (Guangdong Foundation for Program of Science and Technology Research)
- 2022A1515011534/Guangdong Provincial Applied Science and Technology Research and Development Program (Guangdong Foundation for Program of Science and Technology Research)
- 82173239/National Natural Science Foundation of China (NSFC)
- 82100626/National Natural Science Foundation of China (NSFC)
- 2023A1515111152/Guangdong Provincial Applied Science and Technology Research and Development Program (Guangdong Foundation for Program of Science and Technology Research)
- 2021A1515110759/Guangdong Provincial Applied Science and Technology Research and Development Program (Guangdong Foundation for Program of Science and Technology Research)
- 2023A1515011187/Guangdong Provincial Applied Science and Technology Research and Development Program (Guangdong Foundation for Program of Science and Technology Research)
- 2024A1515010739/Guangdong Provincial Applied Science and Technology Research and Development Program (Guangdong Foundation for Program of Science and Technology Research)
- 82472904/National Natural Science Foundation of China (NSFC)
- 2024MS006/Guangzhou Science, Technology and Innovation Commission (Bureau of Science and Information Technology of Guangzhou Municipality)
- 2023MS003/Guangzhou Science, Technology and Innovation Commission (Bureau of Science and Information Technology of Guangzhou Municipality)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

