Dynamic Assembly of Pentamer-Based Protein Nanotubes
- PMID: 39993171
- PMCID: PMC11912573
- DOI: 10.1021/acsnano.4c16192
Dynamic Assembly of Pentamer-Based Protein Nanotubes
Abstract
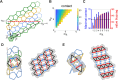
Hollow proteinaceous particles are useful nanometric containers for delivery and catalysis. Understanding the molecular mechanisms and the geometrical theory behind the polymorphic protein assemblies provides a basis for designing ones with the desired morphology. As such, we found that a circularly permuted variant of a cage-forming enzyme, Aquifex aeolicus lumazine synthase, cpAaLS, assembles into a variety of hollow spherical and cylindrical structures in response to changes in ionic strength. Cryogenic electron microscopy revealed that these structures are composed entirely of pentameric subunits, and the dramatic cage-to-tube transformation is attributed to the moderately hindered 3-fold symmetry interaction and the imparted torsion angle of the building blocks, where both mechanisms are mediated by an α-helix domain that is untethered from the native position by circular permutation. Mathematical modeling suggests that the unique double- and triple-stranded helical arrangements of subunits are optimal tiling patterns, while different geometries should be possible by modulating the interaction angles of the pentagons. These structural insights into dynamic, pentamer-based protein cages and nanotubes afford guidelines for designing nanoarchitectures with customized morphology and assembly characteristics.
Keywords: bionanotechnology; cryo-EM; geometry; non-quasi-equivalent; protein cage.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Diversification of Protein Cage Structure Using Circularly Permuted Subunits.J Am Chem Soc. 2018 Jan 17;140(2):558-561. doi: 10.1021/jacs.7b10513. Epub 2018 Jan 2. J Am Chem Soc. 2018. PMID: 29257675
-
[Structure and Self-assembly of Negatively Supercharged Protein Cages].Yakugaku Zasshi. 2019;139(2):199-208. doi: 10.1248/yakushi.18-00169-2. Yakugaku Zasshi. 2019. PMID: 30713229 Review. Japanese.
-
Tailoring lumazine synthase assemblies for bionanotechnology.Chem Soc Rev. 2018 May 21;47(10):3543-3557. doi: 10.1039/c8cs00154e. Chem Soc Rev. 2018. PMID: 29714396 Review.
-
Structure and assembly of scalable porous protein cages.Nat Commun. 2017 Mar 10;8:14663. doi: 10.1038/ncomms14663. Nat Commun. 2017. PMID: 28281548 Free PMC article.
-
X-ray structure analysis and crystallographic refinement of lumazine synthase from the hyperthermophile Aquifex aeolicus at 1.6 A resolution: determinants of thermostability revealed from structural comparisons.J Mol Biol. 2001 Mar 9;306(5):1099-114. doi: 10.1006/jmbi.2000.4435. J Mol Biol. 2001. PMID: 11237620
References
-
- Seeman N. C.; Sleiman H. F. DNA nanotechnology. Nat. Rev. Mater. 2017, 3 (1), 17068.10.1038/natrevmats.2017.68. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

